The Grow Awards 2026 🏆 
























"360" C#10

FLO
Solacure280 Fluorescent/30W

Cond
Hisense
14,000btu
Soil
soil
Indoor
Room Type
Defoliation
weeks 16, 18
Topping
weeks 5-6
Apical dominance
weeks 9
379 liters
Pot Size
Start at Harvest
G
Germination2y ago
Ultraviolet Aloe vera is ideal as a rooting powder alternative because it contains glucomannans, amino acids, sterols, and vitamins. Studies show that these help many types of species develop more and stronger roots when growing cuttings or propagating via air layering.
The ancient tradition of Sacred Geometry is still alive and well in the person of Frank Chester. He has discovered a new geometric form that unites the five Platonic solids and provides some startling indications about the form and function of the human heart. This new form, called the Chestahedron, was discovered in 2000, and is a seven-sided polyhedron with surfaces of equal area. Frank has been exploring the form and its significance for over a decade, His work has potential implications across a number of areas, from physiology to architecture, sculpture, geology, and beyond.
Organic cotton stands out with a frequency of 100Hz, mirroring the human body's frequency.
It's all about the salt (electrolyte).
https://www.seafriends.org.nz/oceano/seawater.htm
.
Plants need elements for normal growth. Three of them--carbon, hydrogen, and oxygen--are found in air and water. The rest are found in the soil.
Six soil elements are called macronutrients because they are used in relatively large amounts by plants. They are nitrogen, potassium, magnesium, calcium, phosphorus, and sulfur.
Eight other soil elements are used in much smaller amounts and are called micronutrients or trace elements. They are iron, zinc, molybdenum, manganese, boron, copper, cobalt, and chlorine. They make up less than 1% of the total but are nonetheless vital.
Most of the nutrients a plant needs are dissolved in water and then absorbed by its roots. In fact, 98 percent are absorbed from the soil-water solution, and only about 2 percent are actually extracted from soil particles.
on that note, some points of interest regarding Boron.
Boron-sugar complexes are formed when boron (B) atoms bond with sugar molecules (saccharides) or sugar alcohols, creating what are known as sugar-borate esters (SBEs).
Boron plays a role in the formation of sugar complexes for translocation within plants, and in the formation of proteins.
Boron aids in the production of sugars and carbohydrates, and is essential for seed and fruit development.
Boron enhances the uptake of calcium, magnesium, and potassium, and enables sugar translocation.
Boron and Plant Nutrition:
Boron is an essential micronutrient for plant growth, and deficiencies can lead to decreased water absorption, root growth, and sugar translocation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6073895/
Boron (B) is an essential trace element required for the physiological functioning of higher plants. B deficiency is considered as a nutritional disorder that adversely affects the metabolism and growth of plants. B is involved in the structural and functional integrity of the cell wall and membranes, ion fluxes (H+, K+, PO43−, Rb+, Ca2+) across the membranes, cell division and elongation, nitrogen and carbohydrate metabolism, sugar transport, cytoskeletal proteins, and plasmalemma-bound enzymes, nucleic acid, indoleacetic acid, polyamines, ascorbic acid, and phenol metabolism and transport. This review critically examines the functions of B in plants, deficiency symptoms, and the mechanism of B uptake and transport under limited B conditions. B deficiency can be mitigated by inorganic fertilizer supplementation, but the deleterious impact of frequent fertilizer application disrupts soil fertility and creates environmental pollution. Considering this, we have summarized the available information regarding alternative approaches, such as root structural modification, grafting, application of biostimulators (mycorrhizal fungi (MF) and rhizobacteria), and nanotechnology, that can be effectively utilized for B acquisition, leading to resource conservation. Additionally, we have discussed several new aspects, such as the combination of grafting or MF with nanotechnology, combined inoculation of arbuscular MF and rhizobacteria, melatonin application, and the use of natural and synthetic chelators, that possibly play a role in B uptake and translocation under B stress conditions.
Apart from the data obtained from agricultural reports that prove the involvement of B in plant growth and development, B often results in deficiency or toxicity because it is a unique micronutrient for which the threshold levels of deficiency and toxicity are very narrow [12]. B deficiency and excess are both widespread agricultural problems for higher plants in arid and semi-arid conditions. B deficiency was primarily observed in apples growing in Australia in the 1930s and subsequently reported in more than 132 field crops grown in sandy soils with low pH and organic matter from 80 different countries [28]. Depending on the age and species, plants manifest a wide range of deficiency symptoms, including stunted root growth, restricted apical meristem growth, brittle leaves, reduced chlorophyll content and photosynthetic activity, disruption in ion transport, increased phenolic and lignin contents, and reduced crop yield [1,8,20]. The prevalence of symptoms depends on the severity of the B-deficiency condition because plants show uniform deficiency symptoms on entire leaves but sometimes in the form of isolated patches. Given the immobile nature of B, it usually accumulates in mature leaves, whereas young leaves do not receive sufficient B for proper growth. Thus, the deficiency symptoms first appear on young leaves, including thick, curled, and brittle leaves with reduced leaf expansion; corky veins; interveinal chlorosis; yellow water-soaked spots on lamina; and a short internodal distance, resulting in a bushy plant appearance [14,29,30]. In severe cases, leaf apex necrosis and leaf dieback occur [12]. The expansion of stems and petioles leads to hollow stem disorder in broccoli and stem crack symptoms in celery [1]. However, in tomato, cauliflower, apple, and citrus, scaly surface development with internal and external corking of fruits is a typical feature associated with B deficiency [13,28].
Amino acids improve plant nutrition by affecting soil microbial activity through the production of a beneficial microbial community and nutrient mineralization in the soil solution, thus enhancing micronutrient mobility [84]. Seaweed extract contains several ions, growth regulators, carbohydrates, proteins, vitamins, and polyuronides, including alginates and fucoidans. These polyuronides can form highly cross-linked polymers and condition the soil, thereby improving the water retention and ion uptake capacity within the soil [89]. Kahydrin, a commercial seaweed component, acidifies the rhizosphere by altering the plasma membrane proton pump and secretes H+ ions that change the soil redox condition and make the metal ions available to plants, leading to improved crop production [90]. Turan and Kose [91] applied three seaweed extracts, including Maxicrop, Algipower, and Proton, on grapevine (Vitis vinifera L. cv. Karaerik) to check the ion uptake efficacy under optimal and deficient ion availability. Maximum micronutrient uptake under optimal conditions were observed with no significant difference among the three kinds of extracts. The alteration in uptake of one ion influences the availability of another ion [85], supporting the idea of B uptake through biostimulator application, but this requires further investigation.
https://pubmed.ncbi.nlm.nih.gov/8508192/
https://pubmed.ncbi.nlm.nih.gov/34988929/
8 likes
16 comments
Share
1
Week 1. Vegetation2y ago
7.62 cm
Height
18 hrs
Light Schedule
28 °C
Day Air Temp
6.5
pH
70 %
Air Humidity
24 °C
Night Air Temp
378.54 liters
Pot Size
121.92 cm
Lamp Distance
Nutrients 3

RAW Humic Acid
0.16 mll

Big Foot Mycorrhizae Granular
1.3 mll

Fish and Seaweed
0.25 mll
Ultraviolet The ideal PPFD level for seedlings is between 100-300 micromoles per square meter per second (μmol/m²/s).
This softer lighting mimics the diffused sunlight of early spring, providing enough energy for seedling-stage plants to develop their initial leaves without overwhelming them.
at 48 inches from light sources, the seedlings receive around 150-180μmol/m²/s, as they grow they grow towards the higher levels of ppfd naturally.
Breaking Down Nitrogen Forms & Their Impact:
Forms of Nitrogen:
Nitrogen, comes in three primary forms: ammonium, nitrate, and urea. Ammonium (NH4+) carries a positive charge, nitrate (NH3–)carries a negative charge, while urea ((NH2)2CO) carries no charge.
Natural Processes in Media:
Once these nitrogen forms are introduced into the growing media, natural processes kick in. Bacteria play a vital role, converting urea to ammonium or ammonium to nitrate. This latter conversion releases hydrogen ions, increasing media acidity.
Urea Conversion:
Urea undergoes rapid conversion to ammonium in the soil, usually within two days. Both urea and ammonium are often grouped together and referred to as ammoniacal nitrogen.
When plants absorb nitrogen, they typically release a molecule with the same charge to maintain internal pH. This process can also alter the pH of the media surrounding the roots.
pH Effects of Nitrogen Uptake:
Ammonium (NO4) Uptake and pH:
When plants absorb ammonium, they release hydrogen ions (H+) into the media. This increases the acidity of the media over time, decreasing the pH.
Nitrate (NO3) Uptake and pH:
Plants take up nitrate by releasing hydroxide ions (OH–). These ions combine with hydrogen ions to form water. The reduction in hydrogen ions eventually reduces the media acidity increasing the pH.
Nitrate (NO3) Absorption Variations:
Sometimes, plants absorb nitrate differently, either by taking in hydrogen ions or releasing bicarbonate. Like hydroxide ions, bicarbonate reacts with hydrogen ions and indirectly raises the media pH.
Understanding these processes helps in choosing the appropriate fertilizer to manage media pH. Depending on the nutrients present, the media’s acidity or alkalinity can be adjusted to optimize plant growth.
Risks of Ammoniacal Nitrogen:
Plants can only absorb a certain amount of nitrogen at a time. However, they have the ability to store excess nitrogen for later use if needed.
Nitrate (NO3) vs. Ammonium (NH4):
Plants can safely store nitrate, but too much ammonium can harm cells. Thankfully, bacteria in the media convert urea and ammonium to nitrate, reducing the risk of ammonium buildup.
Factors Affecting Ammonium (NH4) Levels:
Certain conditions like low temperatures, waterlogged media, and low pH can prevent bacteria from converting ammonium. This can lead to toxic levels of ammonium in the media, causing damage to plant cells.
Symptoms of Ammonium (NH4) Toxicity:
Upward or downward curling of lower leaves depending on plant species; and yellowing between the veins of older leaves which can progress to cell death.
Preventing Ammonium (NH4) Toxicity:
When it comes to nitrogen breakdown of a nutrient solution, it’s crucial not to exceed 30% of the total nitrogen as ammoniacal nitrogen. Higher levels can lead to toxicity, severe damage, and even plant death.
Ideal Nitrogen Ratio for Cannabis:
Best Nitrogen (NO3) Ratio:
Research shows that medical cannabis plants respond best to nitrogen supplied in the form of nitrate (NO3). This helps them produce more flowers and maintain healthy levels of secondary compounds.
Safe Ammonium (NH4) Levels:
While high levels of ammonium (NH4) can be harmful to cannabis plants, moderate levels (around 10-30% of the total nitrogen) are are considered most suitable. This level helps prevent leaf burn and pH changes in the media.
Nitrogen: nitrate (NO3-) and ammonium (NH4+)
Nitrogen is mobile in the plant. When it is in the soil it is mobile as Nitrate NO3– and is immobile as Ammonium NH4+
All those nutrients should be in ionic form, either in the soil or in a nutrient solution. Ions are simply the atomic or molecule form having +ve or –ve charge. As we know, the positive attracts the negative, and the same charge elements will repel each other; this power of charge represents the strength of the element. The positive ions are known as Cation, while negative ions are Anions. The anions want to disperse themselves to even concentrations, so they move from higher concentrations to lower concentrations.
As we look at the soil structure, it’s a composition of particles; those particles attract the positive ions (+Ve), repel the Negative ions (-ve), and float freely in the water. This attraction of Cation by the soil particles is called Cation Exchange Capacity (CEC), which measures the number of cations that can be retained by the soil particles. The higher the CEC, the more Cation Nutrients can be stored in the soil. As a result, the higher CEC soils can become more nutrient-rich; also, keep in mind the soil composition is diverse and varies among different soil types.
7 likes
1 comment
Share
2
Week 2. Vegetation1y ago
10.16 cm
Height
18 hrs
Light Schedule
28 °C
Day Air Temp
6.5
pH
55 %
Air Humidity
24 °C
Night Air Temp
378.54 liters
Pot Size
121.92 cm
Lamp Distance
Nutrients 1
Seaweed Extracts
0.264 mll
6 likes
2 comments
Share
4
Week 4. Vegetation1y ago
20.32 cm
Height
18 hrs
Light Schedule
32 °C
Day Air Temp
7.0
pH
45 %
Air Humidity
27 °C
Night Air Temp
378.54 liters
Pot Size
121.92 cm
Lamp Distance
Nutrients 2
Aluminum Sulphate
1.3 mll

RAW Cane Molasses
5.21 mll
Ultraviolet Surprised by how well the water is wicked to the medium from the water bottle, it took a long time, but wick works as intended. I was unsure how much if any nutrient would be transferred via wick but the high salt content altered pH at certain parts. Right before the symptoms arose I decided to supercrop the stem top to bottom on one plant, shortly thereafter the burning happened from the same plant from running 8.0 pH, and nutrient uptake ground to a halt on the plant that was dealing with stress. She doesn't seem happy.
Remedied with aluminum sulfate, things seem to be coming back to normal.
I need to be more careful with salts. I need MOAR knowledge.
How many weeks shall we veg? Going to take a while to fill that canopy with just two plants, should probably start topping now but I'll wait until pH problem Is remedied first, a couple more days hopefully 🙏.
I defoliated maybe 6 bigger leaves that had burned yellow on one plant, as it comes back to optimal range, this will be a good opportunity to observe the subtle differences that early defoliation can make.
Added a pound of tourmaline powder and biochar, both increase soil urease and invertase activities.
*2025 Didn't charge the biochar skewing my ph drastically.
Urease is an enzyme that catalyzes the hydrolysis of urea into CO2 and NH3 and is a key component in the nitrogen cycle in soils.
Invertase (d-fructofuranosidfructohydrolase, EC 3.2.1.26) is the enzyme that capable both break down α-1,4-glycosidic linkage between d-glucose and d-fructose of sucrose and transferring αβ-D-O-fructofuranoside residue to an acceptor substrate (Toledo et al., 2019).
Since tourmaline is widely distributed in the natural environment and has many excellent physical and chemical properties including radiating far infrared energy, permanently releasing negative ions, producing an electrostatic field, releasing rare microelements, and stimulating the growth and metabolism of microorganisms and plants, tourmaline had been conducted to alleviate potential environmental pollution. Perfect for how much seaweed I've added just incase.
4 likes
comments
Share
5
Week 5. Vegetation1y ago
38.1 cm
Height
18 hrs
Light Schedule
32 °C
Day Air Temp
7.0
pH
45 %
Air Humidity
27 °C
Night Air Temp
378.54 liters
Pot Size
121.92 cm
Lamp Distance
Nutrients 5
KatyayaniSeaweed Extract
1.3 mll
Atlantis 3x Kelp Seaweed Extract
1.3 mll

RAW Amino Acids
1.3 mll
Ultraviolet The mind grows from observing failed attempts,
The body grows from observing failed attempts,
Topped mid-week both plants
Through want of skill and reason's light
Men stumble at noon day;
Whilst busily our Stone they seek,
That lieth in the way.
Who thus do seek they know not what
Is it likely they should find?
Or hit the mark whereat they aim
Better than can the blind?
No, Hermes' sons for Wisdom ask,
Your footsteps she'll direct:
She'll Nature's way and secret cave
And Tree of Life detect.
Son and Moon in Hermes' vessel
Learn how the colors show;
The nature of the elements,
And how the daisies grow.
Great Python how Apollo slew,
Cadmus his hollow oak:
His new raised army, and Jason how
The fiery steers did yoke.
The eagle which aloft doth fly
See that thou bring to ground,
And give unto the snake some wings,
Which in the earth is found.
Then in one room sure bind them both,
To fight till they be dead,
And that a Prince of Kingdoms three
Of both them shall be bred.
Which from the cradle to his crown
Is fed with his own blood;
And though to some it seems strange,
He hath no other food.
Into his virgin mother's womb
Again he enter must;
So shall the King by his new birth,
Be ten times stronger just.
And able is his foes to foil,
The dead he will revive:
Oh, happy man that understands
This medicine to achieve!
5 likes
comments
Share
Used techniques
Topping
Technique
6
Week 6. Vegetation1y ago
45.72 cm
Height
16 hrs
Light Schedule
32 °C
Day Air Temp
6.8
pH
40 %
Air Humidity
25 °C
Night Air Temp
378.54 liters
Pot Size
106.68 cm
Lamp Distance
Ultraviolet Toasty 🔥 Hot
Switched to 16/8,
Surprised by how well she is handling the temperatures.
Direct sunlight in the heat of midday, which can reach UVB levels of 350 to 450 μW/cm2 in tropical settings. However, even the shade has reflected UVB that can reach 30 to 50 μW/cm2.25
I shall try to keep the UV exposure somewhere in between.
The topping turned out nice, with more of an FIM across the crown, this time I hit the sweet spot, on one I managed to cut 4 layers of leaves deep into the mandelbrot crown, once it grows a few days after the cut you really get to see what was actually cut as it takes time to grow out from its miniature form, once tall enough I bent the plant over until it was 6-8 inches from ground. Between the 2 plants we have now 16 stems that should grow in unison given they recieve equal light. The plant having went from a pine 🌲 shape with single top, to a candelabra shape, everything grinds to a halt for what seems like a couple weeks. Reinforcement of stems at 45 degree angles. Plant has to plan for 16 main tops and develop them all evenly as the apical dominance of main stem has been broken. Equal ppfd = equal distribution = equal growth.
I purchased some serious cooling equipment for the tent which will allow me to completely remove temperature as a factor even under high light intensities
First read, sorry its long.
https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.18488
The light environment is crucial for plant growth and development, especially the synthesis and accumulation of anthocyanins, which are affected by spectral components and light intensity (Blancquaert et al., 2019a). Decreases in the ozone layer have led to increased ultraviolet (UV) and infrared (IR) radiation received by plants. The selective absorption of red and blue light by the leaf canopies and the selective transmission of IR and UV radiation enrich the UV and IR radiation in the light environment of the plant. In addition, high-altitude planting areas characterized by high intensity and high proportion of non-visible light wavelengths are increasing, and the prominent color characteristics of the fruits in these areas have already attracted the interest of plant researchers (Mansour et al., 2022). Although there have been reports on the impact of the light environment on anthocyanin biosynthesis in fruits and vegetables, they are mainly focused on the influence of visible light and its underlying mechanisms. In contrast, relevant research on the response mechanism to non-visible light, especially IR radiation, is still relatively scarce. Therefore, in-depth systematic research on the regulatory mechanisms of non-visible light affecting the synthesis and accumulation of anthocyanins is of great significance for adjusting the plant light environment and improving fruit quality.
The color of vegetables and fruits is an important quality indicator, determined by the presence and concentration of specific anthocyanin compounds. Anthocyanin biosynthesis and accumulation are influenced by various environmental factors, among which light and temperature factors play instrumental roles (Liu et al., 2018; Martinez-Luescher et al., 2016). High light intensity, blue/UV light, and low temperature can be applied to promote anthocyanin production in Solanaceous vegetables (Liu et al., 2018). Visible light is mainly involved in photosynthesis, whereas non-visible light plays key roles in the synthesis and accumulation of phenolic compounds, especially anthocyanins (Fernandes De Oliveira & Nieddu, 2016). This review focuses on UV and IR radiation, and by assessing blocking and artificial irradiation experiments, it aims to elucidate the promoting effect of non-visible light on anthocyanin biosynthesis and accumulation in fruits. Moreover, it demonstrates that different non-visible light intensities can alleviate the inhibitory effect of low temperature and high temperature stress on the synthesis of anthocyanins. Transcriptome and metabolome analysis revealed that UVA and IR radiation significantly induce genes related to the flavonoid synthesis pathway and metabolites, promoting the synthesis of anthocyanins (Yin, Wang, Wang, et al., 2022; Yin, Wang, & Xi, 2022).
2 THE CHARACTERISTICS OF NON-VISIBLE SPECTRA
Light, in the form of electromagnetic waves, is the radiant energy emitted by the sun and projected onto the Earth. Its wavelength can range from 100 nm, corresponding to X-rays, to 100 m, corresponding to radio waves (Maverakis et al., 2010). The wavelength range of solar radiation reaching the Earth's surface is 280–2500 nm. However, wavelengths shorter than 280 nm and longer than 2500 nm are absorbed by atmospheric molecules such as ozone and water vapor and cannot reach the ground (Maverakis et al., 2010). The solar radiation absorbed by the ground is reflected back into space as thermal energy, with a proportion of it being blocked by greenhouse gases and reflected back to the ground (Figure 1). The wavelength range of the light spectrum and the respective plant photoreceptors or photosynthetic pigments are shown in Figure 1. Most studies on solar radiation have focused solely on photosynthetically active radiation (PAR), with limited research conducted on non-visible spectra such as UV and IR radiation. UV radiation, which is divided into three types based on wavelength [UVC (100–280 nm), UVB (280–320 nm), and UVA (320–400 nm)], is mainly absorbed by the stratospheric ozone layer (Semenova et al., 2022; Yang et al., 2018). However, UVA and a small portion of UVB can reach the Earth's surface and be absorbed by plants (Loconsole & Santamaria, 2021). UVA radiation accounts for approximately 95% of the UV radiation reaching the Earth's surface (Rai et al., 2021). When harvesting light, plants’ photosynthetic organs are inevitably exposed to relatively high dose of non-visible spectra, including UV and IR radiation (Koyama et al., 2012). Plants can also sense short-wavelength spectra, including UVA and UVB, in addition to the blue and red/far-red light in the visible spectra (Xu & Zhu, 2020). Because of the high diffusive capacity of the UVB radiation, the UVB/PAR ratio is significantly lower on the plant canopy parts exposed to full sunlight than on those in the shade. Furthermore, the long-wavelength spectra of near IR radiation (800–2000 nm) are transmitted (less than 50%), reflected (more than 40%), and absorbed (approximately 10%) by plant leaves. These non-visible spectra play a significant role in plant growth and development.
2.1 Photosynthetic pigments
When green photosynthetic plants are subjected to visible radiation with wavelengths corresponding to the absorption spectra of chlorophyll, carotenoids, and phytochromes, they demonstrate unique bioelectric responses (Mironova & Romanovski, 2001). The pigment chlorophyll plays a crucial role in photosynthesis. Previously, only four types of chlorophyll were known—chlorophyll a, b, c, and d. Chlorophyll a is present in all plants, whereas chlorophyll b is mainly found in higher plants. Both of them can only absorb visible light at 400–700 nm, with the strongest absorption capacity at 640–660 and 430–450 nm (Figure 1). Chlorophyll d has a unique absorption peak in the 710 nm IR region and is exclusively discovered in acaryochloris. In 2010, Chen et al. (2010) reported the discovery of chlorophyll f in cyanobacteria, which has an absorption maximum at 706 nm and fluorescence at 722 nm, determined under in vitro conditions. Further studies revealed that charge separation in photosystem (PS) I and II uses chlorophyll f at 745 nm and chlorophyll f (or d) at 727 nm, respectively (Nürnberg et al., 2018). Additionally, chlorophyll f in the PS is advantageous in environments enriched in far-red light (Mascoli et al., 2020). These findings indicate that photosynthesis can be extended further into the IR region. Photosynthesis in plants can increase the accumulation of sugars, which are important precursor substances for synthesizing anthocyanins (Yan et al., 2023; Yin et al., 2024). There is a close relationship between sugars and anthocyanin synthesis (Yin et al., 2024).
2.2 Photoreceptors
According to the current scientific findings, more than three distinct types of light receptors have been identified and characterized. These include phytochromes (phyA and phyB), which are capable of absorbing both red and far-red light; cryptochromes (CRY1), which are responsive to UVA and blue light; and the recently identified UVR8 receptor, which is specifically activated by UVB radiation (Figure 1) (Yang et al., 2018; Zhang et al., 2021). Phytochromes are present in two different forms, Pr (red-light-absorbing phytochrome) and Pfr (far-red-light-absorbing phytochrome), that are photo-interconvertible depending on the light conditions present (Cho et al., 2003). In plant tissues not previously exposed to UVB, UV RESISTANCE LOCUS 8 (UVR8) proteins are present as homodimers, but upon exposure to UVB radiation, they rapidly dissociate into monomerize (Fernández-Milmanda & Ballaré, 2021). Recent research has indicated that UVR8 receptors may be involved in the perception of both UVB and short-wavelength UVA (UVASW315–350 nm) radiation (Rai et al., 2021). UVR8 and cryptochromes have also been demonstrated to function together to regulate gene expression, altering plant cells’ relative sensitivity to UVB, UVA, and blue wavelengths through their interactions (Rai et al., 2021). These findings suggest that different light receptors may be involved in distinct plant regulatory mechanisms under varying UVA and UVB radiation conditions.
2.3 Plant responses to non-visible spectra
Due to global climate change, it has been projected that increased UV radiation is going to be a significant environmental stressor (Smith, 2023). The depletion of stratospheric ozone associated with climate change has led to elevated levels of UV radiation reaching the Earth's surface (Bernhard et al., 2023). Both UVA and UVB radiation, natural components of solar radiation, can cause plant stress and trigger various acclimatory responses mediated by photoreceptors (Badmus et al., 2022). The rapid modulation of UV shielding in plants is influenced by solar UV radiation and is linked to changes in flavonoid biosynthesis and accumulation (Barnes, Tobler, et al., 2016). The activation of phytochromes and cryptochromes in berries promotes the accumulation of flavonoid and non-flavonoid compounds, such as anthocyanins, flavonols, flavanols, phenolic acids, and stilbenes (Veronica Gonzalez et al., 2015). Flavonoids, widely abundant plant secondary metabolites involved in several biological functions, preferentially accumulate in response to UV exposure and are involved directly in UV absorption and possess antioxidant activity (Jaakola & Hohtola, 2010; Neugart et al., 2021). Although solar UV radiation exclusion did not impact proanthocyanidin concentration and composition, it significantly reduced flavonol concentration (Koyama et al., 2012). Furthermore, exposure to UV radiation did not affect ovule production or seed set per flower but decreased pollen production and total seed production per plant by 31% and 69%, respectively (Carlos Del Valle et al., 2020). The Okra (Abelmoschus esculentus) diurnal rhythms were shown to be regulated by UV radiation, resulting in up to a 50% increase (full UV condition compared to UV-excluding condition) in flavonoid content (Neugart et al., 2021). In addition, although high doses of UV radiation are known to reduce yield and quality parameters, low doses of UV may stimulate biomass accumulation and the synthesis of protective compounds that mainly absorb UV (Loconsole & Santamaria, 2021). Therefore, plants can respond and adapt to non-visible spectra through photosynthetic pigments and photoreceptors to control flavonoid synthesis.
3 ANTHOCYANIN METABOLISM
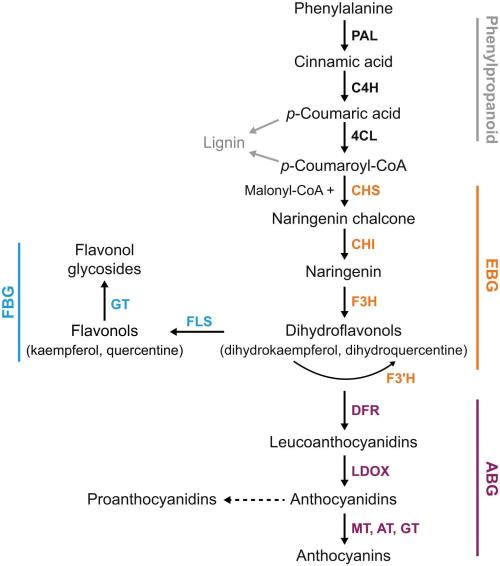
Fruit skin pigmentation is determined by the amount and composition of anthocyanins produced in the cytoplasm and stored in vacuoles as anthocyanin vacuolar inclusions (Azuma, 2018; Flamini et al., 2013). Anthocyanin biosynthesis occurs through the flavonoid biosynthesis pathways, with shared enzymatic steps for the biosynthesis of proanthocyanidins and flavonol derivatives (Sun et al., 2020). Anthocyanin biosynthesis can be divided into three stages: the initial stage involving the phenylpropanoid pathway, the middle stage involving the flavonoid pathway, and the final stage involving the anthocyanin pathway (Figure 2). The mechanisms of anthocyanin biosynthesis and transport in conjunction with photodamage and photoprotection in plants are shown in Figure 2. Initially, glycosides from dihydric anthocyanins, such as cyanidin and peonidin, are accumulated, followed by trihydroxylated anthocyanins, such as delphinidin, petunidin, and malvidin (Downey et al., 2006). B-ring influences the stability of anthocyanin in their structure and the presence of hydroxyl or methoxyl groups (Mattioli et al., 2020). The diversity of anthocyanins is primarily attributed to the activity of O-methyltransferases and anthocyanin acyltransferases, which respectively catalyze the methylation and acylation of anthocyanins (Sun et al., 2020). Anthocyanin transport is facilitated by binding proteins and transporters, such as glutathione S-transferases, ATP binding cassette C family (formerly named multidrug resistance-associated proteins), and multidrug and toxic compound extrusion family (Figure 2) (Sun et al., 2020). Both anthocyanin biosynthesis and transport affect their accumulation in plant tissues.
Currently, four major categories of photoprotective pigments have been identified, namely, mycosporine-like amino acids, phenolic compounds (including phenolic acids, flavonols, and anthocyanins), alkaloids (betalains), and carotenoids (Solovchenko & Merzlyak, 2008). Anthocyanin biosynthesis and accumulation are particularly sensitive to environmental fluctuations that impact their supply and demand, affecting their quantity and chemical variability (Jaakola & Hohtola, 2010). Certain UV and IR radiation spectra or intensities can prove detrimental to plants. Anthocyanins not only function as antioxidants and reactive oxygen species scavengers under osmotic and/or oxidative stress but also offer photoprotection from the epidermis to the mesophyll (as illustrated in Figure 2) (Bao et al., 2022; Kim et al., 2022). Even when not acylated, anthocyanins are able to attenuate visible radiation considerably (Chalker-Scott, 1999). Covalent attachment of the copigment molecule to the anthocyanin results in more effective photoprotection properties than intermolecular anthocyanin-copigment complexes (Da Silva et al., 2012). Anthocyanins can attenuate UV radiation when appropriately acylated with hydroxycinnamic acids (Chalker-Scott, 1999). As a result, non-visible spectra strongly influence anthocyanin formation.
Variations in fruits and vegetables nutrient composition and quality are influenced by environmental factors, including soil conditions, seasonal changes, and climate fluctuations (Askari-Khorasgani & Pessarakli, 2019). Light and temperature are significant factors regulating the biosynthesis of anthocyanins in fruits and vegetables (Liu et al., 2018). The light can be categorized into visible and non-visible spectra. Non-visible spectra are particularly noteworthy for their pivotal role in enhancing anthocyanin synthesis and buildup in fruits and vegetables.
3.1 Non-visible spectra affect anthocyanin biosynthesis
A common plant response to UV exposure is the production of phenolic compounds that absorb damaging light wavelengths (Valenta et al., 2020). The accumulation of UV-absorbing compounds (flavonoids and related phenylpropanoids) in the epidermis of higher plants reduces solar UV radiation penetration to underlying tissues. It is a major acclimation mechanism to changing UV conditions resulting from ozone depletion and climate change (Barnes, Flint, et al., 2016). Anthocyanins are crucial for long-term adaptation to changing illumination conditions and protection against multiple stresses, particularly photodamage (Askari-Khorasgani & Pessarakli, 2019; Solovchenko & Merzlyak, 2008). Plant cultivation at high altitudes poses a challenge due to the increased ratio of non-visible spectra, as the effects of increased UV radiation can lead to enhanced vegetable and fruit pigmentation due to an increase in the synthesis of anthocyanins, flavonols, and tannins (Karagiannis et al., 2020; Mansour et al., 2022). In vivo screening of anthocyanins and carotenoids in leaves has been shown to mitigate the harmful effects of UV stress (Pfuendel et al., 2007; Wang et al., 2019). Anthocyanin biosynthesis and accumulation under UV radiation vary between plant species. Most plants are well-equipped to defend against UV stress at regular altitudes. However, this changes at high altitudes where atmospheric gases and water vapor are inadequate to prevent radiation from reaching plants (Saini et al., 2020). The acylated anthocyanin compounds exhibit efficient mechanisms for rapidly converting the absorbed excitation energy into heat, making acylation a simple yet elegant way for the plant to strengthen its defense mechanisms and capacity against excess UV radiation (Da Silva et al., 2012). The leaves and stems of dropwort (Oenanthe stolonifera) plants exposed to UVA, UVB, and UVC became more red in color compared to the control plants (Jeon et al., 2018). Anthocyanin biosynthesis and accumulation under UV radiation also depend on the plant's developmental stages. The monomers cyanidin and delphinidin exhibited the greatest concentration increase in response to pre and postharvest UV radiation in the turning blueberries (Vaccinium corymbosum L.) fruit ripening stage (Yang et al., 2018). UV radiation significantly impacted the young berries compared to mature berries (Yang et al., 2018). Del-Castillo-Alonso et al. (2021) found that the pea-size and harvest phenological stages exhibited the most significant responses to UV in grapes (Vitis vinifera L. cv. Tempranillo), with the berry skin being the most UV-responsive grape tissue (Del-Castillo-Alonso et al., 2021). Furthermore, preharvest UVB, UVC, and postharvest UVA, UVB, and UVC irradiation significantly promoted blueberries (V. corymbosum L.) anthocyanin biosynthesis, especially the expression of late biosynthesis genes VcDFR, VcANS, VcUFGT, and the transcription factor VcMYB, as well as increased DFR and UFGT activities in a developmental stage and UV wavelength-dependent manner (Yang et al., 2018). When UV wavelengths were excluded, Silene littorea anthocyanin concentrations significantly decreased in petals, stems, and calyces (Carlos Del Valle et al., 2020). At the same time, UV exclusion did not affect the transcript levels of proanthocyanidin-related genes but significantly decreased flavonol-related genes in Cabernet Sauvignon grape (Koyama et al., 2012). There are commonalities in plant responses to UV radiation. However, the differences and specificities in response profiles should not be disregarded.
3.2 Anthocyanins production in response to various UV spectra
The responses of fruits to the various UV radiation types exhibit significant differences (Table 1). Although the UVB radiation effects on plants have been extensively investigated, UVA radiation has been comparatively understudied (Rai et al., 2021). UVA radiation was shown to only slightly increase anthocyanin content in green butter lettuce (Lactuca sativa cv.), whereas it did not significantly impact shoot growth or leaf pigment concentration in Chrysobalanus icaco (Li et al., 2020; Nissim-Levi et al., 2003). However, UVA supplementation has been found to increase flavonoid, polyphenol, and anthocyanin contents in lettuce (He et al., 2021). Grape berries exhibited an increase in anthocyanin content with increasing UVA intensity (Yang et al., 2018). Furthermore, applying UVA radiation in young berries resulted in a more pronounced response in terms of anthocyanin content and accumulation rate (Yang et al., 2018; Yin, Wang, Wang, et al., 2022). On the other hand, increasing UVC intensity has been found to initially increase anthocyanin content in young grape berries, followed by a gradual or immediate decrease (Yang et al., 2018). In sweet basil, UVC radiation had the most significant effect on anthocyanin content, with a 50% increase observed compared to 27% and 0% after UVA and UVB radiation treatment, respectively (Semenova et al., 2022). Ambient UVB levels have been found to have stronger effects than ambient UVA in increasing grape flavonol contents (particularly quercetins and kaempferols) and the expression of flavonol synthase and chalcone synthase genes (VvFLS4 and VvCHS1) (Del-Castillo-Alonso et al., 2021). UVB radiation is absorbed or screened by phenols and flavonoids to protect plant cells from its harmful consequences, and as a consequence, an upregulation of flavonol and anthocyanin biosynthesis is observed (Cechin et al., 2012; Grifoni et al., 2008; Martinez-Luescher et al., 2016). Young grape berries exhibit an initial increase in anthocyanin content with increasing UVB intensity, followed by a gradual or immediate decrease (Yang et al., 2018). Negative feedback loops on the action of UVR8 and cryptochromes can arise from gene expression, signaling crosstalk, and absorption of UV photons by phenolic metabolites (Rai et al., 2021). Therefore, based on the research evidence listed here, UVA, UVB, and UVC play a positive role in anthocyanin biosynthesis.
3.3 UVR8 mediates the UVB response signaling for the induction of anthocyanin biosynthesis
Recent studies have shed light on the UVR8 mediated UVB signal transduction pathways, including UVR8-COP1, UVR8-WRKY36, UVR8-BES1/BIM1, UVR8-HY5/HYH, UVR8-RUP1/2, and UVR8-phytochrome-interacting factor (PIF4) (Brown et al., 2005; Liang et al., 2019; Yao et al., 2020). Among them, COP1 and HY5 are indispensable components of the UVB signaling pathway (Yao et al., 2020). Moreover, the canonical negative regulator in response to visible light, COP1, acts as a positive regulator during UVB exposure (Jin & Zhu, 2019). The last 17 amino acids (C17) in the protein tail of the UVR8 photoreceptor inhibit UVB signaling by attenuating the binding between the C27 domain and COP1 (Lin et al., 2020). Supplementary illumination with UVB radiation increases the affinity of UVR8 to COP1, thereby outcompeting HY5 from interacting with COP1, which results in the accumulation of HY5, promoting the photomorphogenesis of young seedlings (Wang & Lin, 2019). HY5 is a key effector of the UVR8 pathway and is required for anthocyanin biosynthesis under UVB radiation. HY5 binds to the decreased wax biosynthesis promoter elements to repress its expression, promoting anthocyanin biosynthesis in Arabidopsis (Arabidopsis thaliana), thereby affecting plant survival under UVB irradiation stress (Saini et al., 2020). UVR8 interacts with MYB transcription factors (MYB13, MYB 73, and MYB 77) in a UVB dependent manner (Xu & Zhu, 2020). Further genetic and phenotypic observations demonstrate that MYB13 is required for cotyledon expansion and flavonoid biosynthesis in response to UVB exposure (Xu & Zhu, 2020). Exposure to broadband UVB downregulates BES1 expression, thus promoting flavonol accumulation by enhancing the expression of AtMYB11. AtMYB12 and AtMYB111 activate flavonol biosynthesis (Liang et al., 2020). CaMYB113 was shown to interact with CabHLH143 and CaHY5 based on yeast two-hybrid assays, and these three genes may participate collaboratively in UVB-induced anthocyanin biosynthesis in pepper fruit (Wang et al., 2022). Virus-induced gene silencing demonstrated that fruit peels of CaMYB113-silenced plants were unable to turn purple under UVB irradiation (Wang et al., 2022). UVB exposure upregulated the expression of VcPAL, VcCHS, VcF3’H, VcBBX, VcMYB21, and VcR2R3MYB in blueberry fruits (Nguyen et al., 2017). MdWRKY72 promotes MdMYB1 expression both indirectly and directly via binding to a W-box element in the MdHY5 promoter and the MdMYB1 promoter, respectively, to increase anthocyanin synthesis under UVB radiation (Hu et al., 2020). UVR8 is a UVB specific signaling component that orchestrates the expression of a range of genes with vital UV-protective functions, including the induction of the phenylalanine pathway, resulting in the further accumulation of polyphenols, especially anthocyanins, in response to UVB radiation (Brown et al., 2005). Therefore, UVR8 is a key receptor protein regulating plant responses to UVB radiation.
3.4 Anthocyanin accumulation in response to various IR spectra
Among the solar radiation reaching the Earth's surface, UV radiation accounts for only about 3%, visible light accounts for 44%, and IR radiation accounts for 53% (Loconsole & Santamaria, 2021; Rai et al., 2021). In particular, IR radiation not only serves as a form of light signal but also has the ability to generate heat. Current research on IR radiation mainly focuses on its heating effects, which are primarily used in postharvest processing of agricultural products such as drying processes for blueberries and grape seeds (Adak et al., 2017; Fu et al., 2019). IR radiation is also widely applied in quality inspection and breeding of agricultural products. However, research on the role of IR radiation in physiological and biochemical processes in plants, as well as improving fruit quality, is still relatively scarce. Consequently, a comprehensive examination of the available red-to-far red (R/FR) light ratio studies on plants may provide valuable insights into IR radiation function in plants. A high R/FR light ratio induced anthocyanin accumulation in A. thaliana, alpine, and prairie plants (Stellaria longipes) (Alokam et al., 2002; Kim et al., 2022). A low R/FR light ratio induced the expression of CmMYB4, which suppressed the anthocyanin activator complex CmMYB6-CmbHLH2, leading to a reduction in anthocyanin accumulation in Chrysanthemum (Chrysanthemum morifolium) petals (Zhou et al., 2022). On the other hand, under a high R/FR light ratio, CmbHLH16 was upregulated, impeding the formation of the CmMYB4-CmTPL complex and releasing the suppression of CmbHLH2, thus promoting anthocyanin accumulation in Chrysanthemum petals (Zhou et al., 2022). These results suggest that anthocyanin accumulation can be influenced by far-red light. Recently, Yin, Wang, & Xi (2022) demonstrated that in the absence of IR radiation, the anthocyanin content was decreased, whereas the opposite was observed the presence of IR radiation, which increased anthocyanin content (Yin, Wang, & Xi, 2022). The anthocyanin acylation was also found to be affected by IR radiation (Yin, Wang, & Xi, 2022). In the near IR radiation region (800–2000 nm), plant leaf transmittance is less than 50%, the reflectance exceeds 40%, and the absorption rate is 10%. Nonetheless, Mascoli et al. (2020) found that despite the lower energy output, the insertion of redshifted chlorophyll f (whose absorption wavelength can extend up to 750–800 nm) in the PSs remains advantageous in environments that are enriched in FR light and therefore represents a viable strategy for extending the PAR in plants (Mascoli et al., 2020). When the plant IR radiation reflection coefficient is reduced, plant growth and development are limited, negatively affecting yield (Michalak et al., 2018). Sunlight contains a considerable proportion of IR radiation, which has heating and light-signaling effects on plants.
4 NON-VISIBLE SPECTRA × TEMPERATURE INTERACTION
4.1 Non-visible spectra × high temperature interaction
As global warming persists, incidents of sunburn damage (due to both high light and temperature) in vineyards are becoming more frequent, leading to the destruction of photosynthetic pigments and the accumulation of polyphenols (Gambetta et al., 2022). Carotenoid pigments, such as orange carotenoid protein (OCP), convert excess light energy into heat (Hamant, 2021). OCP can regulate fluorescence in light, temperature, and other types of sensors in cyanobacteria (Muzzopappa & Kirilovsky, 2020). The anticipated rise in average temperatures is projected to impact plant phenological stages differently based on the temperature gradient, with warmer areas experiencing a greater acceleration of phenological stages, particularly veraison and maturity, which appear earlier (Ramos & Martinez de Toda, 2020). At harvest, a negative correlation between anthocyanin content and ambient temperature has been observed (Gutierrez-Gamboa et al., 2021; Yin et al., 2023). High temperatures can lead to the hydrolysis of anthocyanins, producing methanol pseudoalkaloid (Ramos & Martinez de Toda, 2020). The higher the temperature, the faster the degradation rate of anthocyanins, ultimately resulting in the discoloration of anthocyanins. Although ambient temperature increases may reduce plant protection by decreasing the UVB-mediated accumulation of phenolics, other defense-related compounds have been shown to increase under such elevated temperature conditions (Escobar-Bravo et al., 2017). Micrometeorological changes shift the balance between the most abundantly accumulated flavonoids, with increased solar exposure associated with lower levels of anthocyanins and flavan-3-ols and a higher flavonol accumulation (Reshef et al., 2018). UV radiation and high temperature stimulate anthocyanin acylation in the Bovale grande grape cultivar, particularly toward the formation of coumaroylglucosides (Fernandes de Oliveira & Nieddu, 2016). Both temperature and light have a synergistic effect on the expression of the anthocyanin biosynthesis pathway genes, which determines anthocyanin accumulation (Azuma, 2018). The expression of anthocyanin biosynthesis genes VvMYBA1, VvGST, VvOMT2, and VvCHS2 increases under elevated UVB and temperature conditions (Martinez-Luescher et al., 2016). A simplified schematic representation of the main TFs/genes involved in the regulation of anthocyanins under high temperature and UVB radiation. The expression of MdCOL4 is reduced by UVB but promoted by high temperature (Fang, Dong, Yue, Chen, et al., 2019). MdCOL4 interactes with MdHY5 to synergistically inhibit the expression of MdMYB1 or directly binds to the promoters of MdANS and MdUFGT, which encode genes in the anthocyanin biosynthesis pathway, to suppress their expression (Fang, Dong, Yue, Chen, et al., 2019). Therefore, the effect of UVB compensates for the deleterious effect of increased temperature on berry anthocyanin concentration (Martinez-Luescher et al., 2016). The negative role of high temperature in anthocyanin accumulation can be reduced by application of non-visible spectra.
4.2 Non-visible spectra × low temperature interaction
Non-visible spectra have also been shown to interact with low temperatures in the regulation of anthocyanin biosynthesis (Sytar et al., 2018). As shown in Table 2, UVB radiation was more effective at inducing anthocyanin synthesis in peel tissues and improving fruit coloration at 27°C than at 17°C (Zhang et al., 2012). A lower temperature of 10°C during UVB + visible light irradiation prevented anthocyanins and quercetin glycoside accumulation in apple fruit skin compared to 20°C (Reay & Lancaster, 2001). Acclimation to low temperatures was also shown to increase PS II sensitivity to UVB radiation (Schultze & Bilger, 2019). The regulation of light and cold signaling in plants is coordinated by the photoreceptor and thermosensor phyB, as well as the transcription factors PIFs and CBFs, which form complex regulatory networks (Xu & Deng, 2020). PIF3, a basic helix–loop–helix transcription factor, plays a critical role in light signaling and was shown to negatively regulate freezing tolerance in Arabidopsis (Lin et al., 2018). As shown in Figure 3, which describes a model of UVR8-mediated signaling, the apple B-box protein, MdCOL11, is involved in UVB and low temperature-induced anthocyanin biosynthesis (Bai et al., 2014). Furthermore, MdBBX20 was shown to interact with MdHY5 in vitro and in vivo, which greatly enhanced the promoter activity of MdMYB1 and induced anthocyanin biosynthesis (Fang, Dong, Yue, Hu, et al., 2019). MdBBX20 was also responsive to low temperatures (14°C) with the involvement of MdbHLH3, which directly binds to low temperature response cis-elements in the MdBBX20 promoter (Fang, Dong, Yue, Hu, et al., 2019). The levels of UV radiation predominantly influence the accumulation of flavonoids and anthocyanins, whereas temperature plays a more significant role in the accumulation of phenolic acids (Sytar et al., 2018). Low temperatures more strongly influence the expression of CaMYB, CaF3’5’H, CaDFR, and CaANS than UVB radiation in bell pepper (Gerardo Leon-Chan et al., 2020). The expression of PyMYB10 and five anthocyanin structural genes, PpPAL, PpCHI, PpCHS, PpF3H, and PpANS, were also higher in fruits irradiated with UVB at 27°C than at 17°C (Zhang et al., 2012). The expression of MdCHS, MdF3H, MdDFR, MdANS, and MdUFGT was enhanced by UVB and low temperature (17°C) treatments, resulting in the accumulation of anthocyanins in the apple fruit skin (Ubi et al., 2006). Therefore, UV radiation can enhance cold tolerance and relieve the repression of low temperatures on anthocyanin biosynthesis.
5 CONCLUSION
The accumulation of UV-absorbing compounds in the epidermis of higher plants is a primary mechanism of acclimation to changing UV conditions resulting from ozone depletion and climate change. Anthocyanins are crucial in protecting plants against multiple stresses, particularly photodamage caused by increased UV radiation. In vivo protection by anthocyanins and carotenoids can mitigate the effects of UV stress in the leaves. The biosynthesis and accumulation of anthocyanins under UV irradiation depend on the genotype, organism, and the developmental stage. Different UV radiation types affect fruit growth and pigment content differently. UVA radiation has been observed to slightly increase anthocyanin content in some plants, whereas it was shown to improve flavonoid, polyphenol, and anthocyanin contents in lettuce. On the other hand, increasing UVC intensity initially increases anthocyanin content but could lead to a gradual or immediate decrease. UVB radiation is absorbed or screened by phenols and flavonoids to protect against its harmful consequences and upregulate flavonol and anthocyanin biosynthesis. Recent studies have identified several UVR8-mediated UVB signal transduction pathways, including UVR8-COP1 and UVR8-HY5, as key components of UVB signaling. HY5 is a key effector of the UVR8 pathway, promoting anthocyanin biosynthesis and affecting plant survival under UVB irradiation stress. UVR8 orchestrates the expression of genes with vital UV-protective functions, resulting in the increased accumulation of polyphenols, especially anthocyanins, in response to UVB radiation.
Although various research studies have examined the impact of UV irradiation on plants, research on the application of IR radiation to plants is limited. However, recent research has shown that anthocyanin content is reduced in the absence of IR radiation but enhanced in the presence of IR radiation. Moreover, IR radiation was shown to affect the acylation of anthocyanins. Decreasing the IR radiation reflection coefficient can limit plant growth and development, negatively affecting yield. Drawing on the research results of red light and far-red light, theoretical foundations and directions for the research on IR radiation can be provided. Further research on the photoreceptors of IR radiation and the study of their physiological effects on plants under conditions of heat isolation.
Both non-visible spectra and temperature influence anthocyanin accumulation in fruits and vegetables. UVB radiation effectively induces anthocyanin synthesis and improves fruit coloration at higher temperatures. Furthermore, UVB radiation relieves the low temperature repression effect on anthocyanin biosynthesis. Several photoreceptors and transcription factors coordinate the regulation of light and cold signaling. Transcription factors from various families, including C3H, MYB, BBX, bHLH, and WRKY, may contribute to color differences in fruits and vegetables. Therefore, exposure to certain non-visible spectra, such as UV and IR radiation, can significantly increase the anthocyanin content in certain crops. The information covered here and their interpretation will contribute to a complete understanding of how environmental factors affect coloration, enabling growers to develop cultivation practices that contribute to the consistent production of uniform, high-quality fruits and vegetables. In the future, we should place more emphasis on the exploitation and utilization of non-visible light, identify the optimal wavelengths for specific plants and developmental stages, and provide ideas for efficient and sustainable food production in the horticultural industry.
https://onlinelibrary.wiley.com/doi/full/10.1002/fft2.426
6 likes
comments
Share
Used techniques
Topping
Technique
7
Week 7. Vegetation1y ago
30.48 cm
Height
16 hrs
Light Schedule
28 °C
Day Air Temp
6.8
pH
40 %
Air Humidity
22 °C
Night Air Temp
378.54 liters
Pot Size
106.68 cm
Lamp Distance
Ultraviolet ...
3 likes
comments
Share
8
Week 8. Vegetation1y ago
30.48 cm
Height
16 hrs
Light Schedule
28 °C
Day Air Temp
6.8
pH
40 %
Air Humidity
22 °C
Night Air Temp
378.54 liters
Pot Size
106.68 cm
Lamp Distance
Nutrients 1
Aluminum sulfate.
1.3 mll
Ultraviolet ttttttttt
3 likes
comments
Share
9
Week 9. Vegetation1y ago
45.72 cm
Height
16 hrs
Light Schedule
28 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
22 °C
Night Air Temp
378.54 liters
Pot Size
101.6 cm
Lamp Distance
1200 PPM
CO₂ Level
Nutrients 1
Gibberellic Acid
0.75 mll
Ultraviolet Still got some slight burning of leaf tips more so on the right side of the tent. Otherwise, growth is good.
Stratiolaelaps scimitus are now abundant in the soil. Pretty neat.
GIBBERELLIN:
Note: As gibberellic acid is not soluble in water you need to dissolve in alcohol first,
Mixed 0.75g Gibberellic Acid 90% with 50ml of 99% Isoprponal alcohol , (Any alcohol will do but a higher % works better) also warmer alcohol helps dissolve the gibberellin.
Mixed with 1000ml =1L of distilled water, bringing the concoction to 400ppm. 2000ml = 200ppm etc
Every plant has a different threshold required to elicit a response, 400ppm is on the high end of the scale I've read to be careful with dosage as it's easy to over-apply, let's find out if it does anything like it described.
Added bunch of enzymes, aminos, 5 or so different kelp.
Can be kept for 1 week in the refrigerator.
Nutrients for the week are recipe foliar application. The speaker (containing a 3 KHz signal and nature sounds) is played at high volume with high treble and medium bass for 10 minutes before spraying the plants. The plants are then sprayed while it's playing and the sound is continued for another 20 minutes after spraying. Both sides of the leaves should be saturated. Treatment is best performed early in the morning/daylight, preferably in foggy 65+RH% dew. On cold mornings, spraying should be delayed until late afternoon if outdoors. Do not spray plants when the temperature falls below 50o F. The formula also can be administered in the regular weather supply, by drip-feeding, hydroponics, etc.. The nutrient solution should be applied once somewhere in first 4 weeks, then twice weekly thereafter.
https://biologydictionary.net/gibberellin/
Once a plant has sprouted past the surface of the soil, the endosperm is long gone. The plant must now rely on photosynthesis for food. However, the role of gibberellin does not stop at the seed. Gibberellin is responsible for many aspects of plant development. Further, plants produce many forms of gibberellin molecules, which act on different parts of the plant. In the image below, you can see the effects of a specific gibberellin applied to a plant.
In number 1, no gibberellin was applied. Plants 2 and 3 both had gibberellins applied, with plant 3 receiving the highest dose. Gibberellin here encourages the plants to increase their internode length or the length between their leaves. In many plants, the regulation of gibberellin is an important natural process that regulates their height due to this process. At the cellular level, gibberellin is influencing the balance of proteins. In doing so, it encourages cell growth and elongation in the stems and between nodes.
In some species of plants, gibberellin is involved in many more processes. These include flowering, fruiting, and senescence, or the natural death of leaves and other plant parts. Interestingly, many genes that regulate and adjust gibberellin levels are influenced by the temperature. Thus, when the temperature changes during seasonal change, the plants react to this as gibberellin levels change. This starts off many processes such as flowering and fruiting.
Gibberellin molecules are involved with and interact with other plant hormones. The auxin level, for example, is directly related to the gibberellin level, and the two complement each other. Ethylene, on the other hand, tends to degrade gibberellin levels. Plants use these hormones, which respond to different inputs, to balance and react to inputs from the environment. These inputs signal various environmental conditions, which the plant is keen to take advantage of.
Gibberellin Structure
Gibberellin molecules of different types are synthesized in many different parts of the plant. Currently, there are over 100 uniquely identifiable gibberellin molecules. These molecules are synthesized in many cells of the plant, but tend to be concentrated in the roots. This is different from auxin, which tends to concentrate at the apex.
Gibberellin is a diterpenoid, which is a familiar and highly represented molecule in biochemistry. It forms the basis of molecules like Vitamin A and Vitamin E. Seen below is Gibberellin A1, which was the first identified gibberellin.
Other gibberellins have the same basic structure, but have various side groups attached. These groups affect where and how the gibberellin acts, which is how gibberellin can have so many diverse and unique functions in different tissues
3 likes
comments
Share
Used techniques
Apical dominance
Technique
10
Week 10. Vegetation1y ago
45.72 cm
Height
16 hrs
Light Schedule
28 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
22 °C
Night Air Temp
378.54 liters
Pot Size
86.36 cm
Lamp Distance
1500 PPM
CO₂ Level
Ultraviolet Finally getting her dialed in, guess I better mentally prepare for flowering. *slaps face*
All week I kept bending further down, any stem that shot up past a point where it could reach the next square across in any direction that needed to be filled, nice and easy. Tweak tweak tweak all week.
Break Apical dominance
In botany, apical dominance is the phenomenon whereby the main, central stem of the plant is dominant over other side stems; on a branch the main stem of the branch is further dominant over its own side twigs.
Resonance transfer occurs because the energy waveforms coming out of a vibrating substance have nearly identical waveforms. The phase relationships of a resonating system can be defined in terms of the angular separation that exists between adjacent molecules. The six molecules that form one hexagonal ring, when in resonance, will vibrate or broadcast its energy on a wave that has a particular frequency. The first molecule will vibrate influencing the second molecule; the second will affect the third, and so on. The time intervals between successive broadcasts will result in a phase delay which can be converted into a phase angle. Successive waves coming from an oscillating system have successive phase angles that equal the angular separation that exists between members of the system.
The phase angle within the vibrating ring of water molecules is 60 degrees (360 divided by 6). If there were 7 molecules in the ring the phase angle would be 360/7 or 51.43 degrees. This is the principle phase angle of quartz. Most of the internal angles of water and quartz are either fractions of this angle or multiples of it.
It is the interlattice resonance between the quartz microstate and the lyotropic mesophase that is the determining factor in the formation of the lyotropic mesophase in water. It is the reason that water can be structured by spinning it around a tuned quartz crystal. It does not require large amounts of energy to accomplish this.
As an example of resonant transfer, one can imagine a series of dominoes being placed across the United States. from San Francisco to Washington. Each successive domino would be slightly larger and heavier than the previous piece. By the time we reached Washington, the final domino might be as large as the Washington monument. By applying a slight push to the first domino, less than one pennyweight, each domino would be knocked over until the final piece was toppled. Through the introduction of a very small energy to a system the result is the production of enough energy to knock over the final very large and heavy domino.
3 likes
3 comments
Share
11
Week 11. Vegetation1y ago
76.2 cm
Height
16 hrs
Light Schedule
28 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
22 °C
Night Air Temp
378.54 liters
Pot Size
86.36 cm
Lamp Distance
800 PPM
CO₂ Level
Ultraviolet Whosoever affirmeth that the Philosophers' grand Secret is beyond the powers of Nature and Art, he is blind because he ignores the forces of Sol and Luna.
Switched to flowering spectrum.
The circumference of the circle is 24π inches, 24 inches of the pie, I love apple pie so much. Gonna go bake me some after i spark up this cone.
Rhythmic exposure to moonlight has been shown to affect animal behavior, but its effects on plants, often observed in lunar agriculture, have been doubted and often regarded as myth. Consequently, lunar farming practices are not well scientifically supported, and the influence of this conspicuous environmental factor, the moon, on plant cell biology has hardly been investigated. We studied the effect of full moonlight (FML) on plant cell biology and examined changes in genome organization, protein and primary metabolite profiles in tobacco and mustard plants and the effect of FML on the post-germination growth of mustard seedlings. Exposure to FML was accompanied by a significant increase in nuclear size, changes in DNA methylation and cleavage of the histone H3 C-terminal region. Primary metabolites associated with stress were significantly increased along with the expression of stress-associated proteins and the photoreceptors phytochrome B and phototropin 2; new moon experiments disproved the light pollution effect. Exposure of mustard seedlings to FML enhanced growth. Thus, our data show that despite the low-intensity light emitted by the moon, it is an important environmental factor perceived by plants as a signal, leading to alteration in cellular activities and enhancement of plant growth.
https://www.researchgate.net/publication/374055659_Moonlight_Is_Perceived_as_a_Signal_Promoting_Genome_Reorganization_Changes_in_Protein_and_Metabolite_Profiles_and_Plant_Growth
4 likes
1 comment
Share
12
Week 12. Vegetation1y ago
96.52 cm
Height
12 hrs
Light Schedule
27 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
23 °C
Night Air Temp
378.54 liters
Pot Size
76.2 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 10
Bee pollen
0.33 mll
Honey
0.65 mll
Pottasium Sulphate
0.65 mll
Ultraviolet If you are lonely when you are alone, then you are in bad company.
Bee pollen is considered a “vitamin bomb” due to the presence of almost all vitamins with an average of 0.02–0.7% of its total content, with a higher amount of water-soluble than fat-soluble vitamins. Bee pollen contains vitamins A, D, E, B1, B2, B6, and C. It also provides minerals such as calcium, magnesium, phosphorus, potassium, zinc, copper, manganese, iron, and selenium, I mixed a bunch of that with some honey and RAW cane molasses to make a nice big bucket of tea. A family friend who is a beekeeper was kind enough to share some honey. The nutritional content of raw honey is impressive and includes high levels of protein, amino acids, B vitamins, calcium, manganese, potassium, magnesium, zinc, and iron, as well as various polyphenolic antioxidants.
I am loading up nature's finest sugars, and sweet things, Honey & Mollases.
UV-B-induced DNA damage (CPDs and 6–4 PPs) can be repaired efficiently by photolyases. Pyrimidine dimers can be repaired by nucleotide excision repair (NER), or bypassed by replicative polymerases (Britt 2004). The expression of the CPD photolyase (PHR) gene is induced by UV-B light dependent on UVR8 signaling pathway, and is also induced by blue and UV-A light (Li et al. 2015)
https://link.springer.com/article/10.1007/s44154-022-00076-9?fromPaywallRec=true
Old but gold.
The camera picks up far more light than there is during the night cycle, camera is showing bright pink violet collages but my eyes barely see a thing, about 0.25ppfd in that tent overnight. Have been tweaking the spectrum of moonlight/intensity and watching the responses overnight.
Tweak, tweak, tweak all week.
PAR is 400-700nm,
Overnight UVA in the tent is all 365nm and 385nm, so the meter only picks up a fraction of the light curve that makes it photosynthetically active past 400nm. Of the light in the tent, 0.25ppfd is from UVA
Looks like It makes them 🕺 🕺 💃 all night.
Better flower soon or ill be screwed for space, they are stretching, but is it "the stretch"? She has fire in her belly.
Growing crops with insufficient light (i.e., below “optimal,” as defined here) limits the yield potential, which in turn wastes the other production inputs including labour, water, nutrients and electricity. As lighting fixture is one of the most expensive investment of the production, what is the relationship between light intensity and yield?
Potter and Duncombe (2012) grew cannabis plants with varying canopy-level PPFDs during the flowering stage and found that increasing PPFD from 400 to 900 μmol·m−2·s−1 increased yield an average of 1.3 times higher, across seven cultivars, with no light intensity treatment effects on floral cannabinoid concentrations. Vanhove et al. (2011) found that cannabis yields were 1.3 to 3.1 times higher (depending on cultivar) when plants were grown under approximately 1000 μmol·m−2·s−1 compared to approximately 450 μmol·m−2·s−1 during the flowering stage.It was predicted that cannabis yield would exhibit a saturating response to increasing Light intensity, thereby signifying an optimum light intensity range for indoor cannabis production.
However, a new research from Morrison (2021), after 81 days‘ experiment, found that When plants grew under LI ranging from 1200 to 1800 μmol·m–2·s–1 provided by light emitting diodes (LEDs), inflorescence yield increased linearly as LI increased up to 1800 μmol·m–2·s–1.
"Cannabis will not stop flowering if the lights are turned on for a few minutes once or twice during the 2-month-long flowering cycle. If a light is turned on for 5 to 30 minutes—long enough to disrupt the dark period—on 3 to 5 consecutive nights, plants will start to revert to vegetative growth."
"Less than one half of one foot-candle of light (0.1ppfd) from sunlight will prevent cannabis from flowering. That is a little more light than is reflected by a full moon on a clear night. Well-bred indica-dominant plants will revert within three days. Sativa-dominant plants take four to five days to revert to vegetative growth. Once they start to revegetate, it can take from four to six additional weeks to induce flowering again!"
Guess ill find out my answer soon.
3 likes
comments
Share
13
Week 13. Vegetation1y ago
96.52 cm
Height
12 hrs
Light Schedule
27 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
23 °C
Night Air Temp
378.54 liters
Pot Size
76.2 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 8
Pottasium Sulphate
5.21 mll

RAW Cane Molasses
2.6 mll

RAW Enzymes
0.33 mll
Ultraviolet They are not praying, and they are not even thrilled. What they represent to me is discipline, and being pushed to limits, the disciplined formation of a plant being pushed to its optimum, both photosynthetically and thermally, turgor pressure is spot on, indicating that she is dialed in, cycling efficiently, and ready to switch to flower.
She is stressed; more cohesion = more stress.
RELATIVE HUMIDITY
The term ‘relative humidity’ (RH) refers to the amount of water vapor in the air and is usually expressed as a percentage (e.g. 50% RH). This can have a major impact on how cannabis plants grow. Low humidity means less water in the air and results in increased evaporation and water use. Excessive humidity comes with its own problems, including creating an ideal environment for pests, mildew, and mold to grow.
One key factor related to humidity that is often left out of the conversation is vapor-pressure deficit (VPD) – the difference between the maximum water vapor the air can hold at a given temperature and RH. Although not all growers measure VPD, it significantly influences stomata activity and is directly related with transpiration rate and metabolism.
A VPD that is too high means drier air and increased evaporation and transpiration. Too low a VPD can lead to slowed transpiration and reduced growth. Since slowed transpiration reduces nutrient uptake, both too high and too low of a VPD may appear as nutrient deficiencies. It is VPD that drives transpiration and nutrient uptake in plants; the uptake of water at the roots is determined by the loss of water through the shoots, and the loss of water through the shoots is determined by how much water is in the air.
Humidity levels influence the rate of water evaporation from the leaves of cannabis plants, which directly affects the tension and suction created within the plant. Higher humidity levels can reduce the rate of evaporation, potentially impacting the negative pressure and water transport efficiency within the plant.
CARBON-e DIOXIDE
Carbon dioxide is essential for photosynthesis. Light energy is used to convert CO2 and H2O into sugar and oxygen. As the CO2 concentration increases, the rate of photosynthesis increases until a saturation point where no more CO2 can be absorbed. The guard cells (stomata) previously mentioned are specialized to regulate gas exchange, working to optimize the movement of oxygen, water, and CO2 in and out of the shoots.
Plants cultivated outside typically don’t need supplemental CO2 (because nature knows what it’s doing). Indoor growers however, may find themselves needing additional carbon dioxide to maximize yields and improve plant growth and development. Without fresh air for plants to exchange oxygen for carbon dioxide, the CO2 concentrations can become low, hindering photosynthesis and dramatically reducing plant growth.
Although CO2 is a naturally occurring gas that both humans and plants use, it is invisible and odorless and can be fatal at high-levels. If you’re supplementing carbon dioxide in your grow room, ensure there are no leaks in any CO2 devices and always use a CO2 monitor and alarm.
0.02% Life unsustainable
0.03% Life OK
0.04% Current ambient atmospheric co2
0.04%-0.1% =400-1000ppm standard indoor co2.
0.1%-0.2% =1000-2000ppm (prolonged exposure drowsiness).
0.2%-0.4% = 2000-4000ppm (Headaches, fatigue, stagnant, stuffiness, poor concentration, loss of focus, increased heart rate, nausea).
1% is toxic
5% quick death.
AIRFLOW
Outdoor plants are constantly exposed to natural elements, and that includes wind. Airflow ventilation is one of the often-forgotten environmental factors in healthy cannabis growth and development. Like all environmental factors, we want to “recreate” beneficial stressors that the plant would be exposed to outdoors.
Like human bone that becomes stronger in response to stress from resistance we call exercise, stems increase in rigidity and structural integrity in response to stress from air flow. Plants that lack airflow are prone to developing weak stems, leaving them tall, skinny, and unable to hold bud weight as the plant grows. Excessive air flow, on the other hand, which constantly bends the entire plant, could lead to stunted growth or even broken shoots. Thankfully, you don’t need a wind sensor to achieve optimal air flow; a light breeze that just makes the leaves wave or dance gently can assist in the development of strong, dense shoots. A little too much though can stress so be careful not to overdo it too hard for too long as it will eventually stress.
Stagnant air within the grow space can also increase the risk of pests, mold, and mildew. Some pests hide under leaves, along stems, and even in the soil itself. A small fan providing a gentle breeze is often enough to prevent a stationary environment, build stem strength, and reduce the chance of pests or pathogens.
Proper air circulation and CO2 exchange facilitated by negative pressure contribute to stronger and healthier plants. Good air flow with constant fresh air is essential for maximizing the growth and yield of your indoor plants..
To achieve and maintain negative pressure in your grow tent, several key factors and components come into play. Understanding how these elements work together is essential for creating negative pressure inside your grow tent.Start by selecting an exhaust fan with an appropriate CFM (cubic feet per minute) rating for your specific grow tent size.
The CFM rating determines the amount of air the fan can move per minute, and it’s crucial to choose a fan that can sufficiently exchange the air within the tent to create negative pressure.
Install the exhaust fan at the highest point in the grow tent to effectively remove warm and stale air from the space. Mounting the fan near the top allows it to expel the warm air, which naturally rises.The negative pressure then automatically draws in fresh air from the lower intake points.
Depending on the size and airflow requirements of your grow tent, consider adding a lower intake fan to facilitate controlled air exchange. An intake fan can help regulate the inflow of fresh air and contribute to maintaining balanced pressure within the tent. Want the exhaust higher CFM than lower Intakes, this is what will give us a negative pressure
Slight negative pressure is good for maximizing the yield of a growth regime. It makes it easier to control the temperature, humidity, CO2 levels, and other contaminants of the tent.
Well, too much of everything is always bad. And the same does for negative pressure as well. So, how would you understand whether the negative pressure exceeded the limit?
The simple trick is- if the tent itself seems to pull itself inwards, the negative pressure is still under the tolerable limit. If the pressure gets as high as it bends the poles inwards, that’s where the danger limit starts.
So, if you see the poles bend inwards, the negative pressure is something to worry about. Otherwise, if it’s the tent itself if pulled inwards slightly, you don’t have to worry about it.
The cohesion-tension theory explains how negative pressure enables water movement from the roots to the leaves of a cannabis plant. As water evaporates from the leaf surfaces through stomata, tension is created, generating a suction force that pulls water upwards through the xylem vessels. This process relies on the cohesive forces between water molecules, forming a continuous column for efficient water transport.
In cannabis plants, xylem vessels serve as the conduits for water transport. These specialized cells form interconnected channels that allow water to move upwards from the roots to the leaves. The negative pressure generated through the cohesion-tension mechanism helps drive the water flow within the xylem vessels.
Negative pressure facilitates the movement of water from the soil, through the roots, and up to the leaves of cannabis plants. It helps maintain proper hydration and turgor pressure, ensuring the cells remain firm and upright. This is crucial for healthy growth and structural support.
Negative pressure transports water and aids in the uptake and transport of dissolved nutrients within the cannabis plant. As water is pulled up through the xylem vessels, essential nutrients and minerals are transported along with it, supplying the various tissues and organs where they are needed for optimal growth and development.
ROOTS
OXYGEN
As well as releasing oxygen created during photosynthesis, plants need to absorb oxygen to perform respiration – i.e. to make energy. Since plant roots are non-photosynthetic tissues that can’t produce oxygen, they get it from air pockets in the soil or grow medium. These air pockets can vary in size based on the makeup of the growing medium, and also on the water saturation levels of the medium.
Root oxygenation and soil aeration play an important role in both transpiration and cellular respiration in all plants. This means that plants are highly dependent on the growing medium that holds the optimal amount of oxygen within. Make sure not to overwater, as roots in compacted soil or fully submerged in water with low O2 can cause irreversible damage if left unchecked. This is why even when growing hydroponically, when the roots are submerged in water, it’s important to have an air pump to incorporate adequate O2 to the roots. Grow mediums like coco coir and soils that contain perlite promote aeration and are less prone to overwatering.
TEMPS
Whether it’s sunlight outdoors or artificial lights indoors, when light heats the air temperature, soil temperature also rises. But it’s not only the air that influences the soil temperature; the grow medium, plant depth, and moisture level can also change how well the soil releases or retains heat. Not all growers monitor soil temperature, but roots are the reservoir system of water and nutrients, and if they are the wrong temperature, things can deteriorate quickly for any plant.
Roots are a living part of the plant and therefore have an optimal temperature range in which they thrive at water and nutrient uptake. Although every plant varies, root temperatures above 88°F & below 55°F (above 31°C and below 12°C) can result in stunted growth and ultimately plant death if exposed for too long. 73-76, Avoid going over 77F as common bacterial growth explodes above 77, if disease strikes it's going to strike 10x faster above 77F.
WATER
Water is one of the most important factors of cannabis growth and development; both transpiration and photosynthesis involve water. Irregular watering can lead to irregular plant growth and development. Too little water and your plant can become dry, brittle, and stressed. Too much water and your plant’s roots can be deprived of important oxygen, and even drown. One of water’s most important purposes is the transportation and movement of nutrients and minerals, which are typically absorbed in the roots and distributed throughout the rest of the plant. The faster the plant can rid itself of water through transpiration the faster it can uptake more water to get more nutrients to where they need to be, by creating a negative pressure we optimize turgor pressure increasing nutrient uptake, by sticking to VPD we optimize transpiration rate and maximize stomatal openings, with sound frequency we open them further.
NUTRIENTS
Plant growth and development depends on nutrients derived from the soil or air, or supplemented through fertilizer. There are eighteen essential elements for plant nutrition, each with their own functions in the plant, levels of requirement, and characteristics. Nutrient requirements generally increase with the growth of plants, and deficiencies or excesses of nutrients can damage plants by slowing or inhibiting growth and reducing yield. Many deficiencies can be recognized by observing plant leaves.
When most people hear the word “fertilizer” they think of synthetic fertilizers, but the word fertilizer refers to any substance or mixture added to soil or a growing medium that increases its fertility or ability to sustain life. Some fertilizers are synthetically produced, and others are mixtures of decomposed organic waste such as worm castings or bat guano (aka bat poop), which are rich in essential nutrients.
Plants require eighteen elements found in nature to properly grow and develop. Some of these elements are utilized within the physical plant structure, namely carbon (C), hydrogen (H), and oxygen (O). These elements, obtained from the air (CO2) and water (H2O), are the basis for carbohydrates such as sugars and starch, which provide the strength of cell walls, stems, and leaves, and are also sources of energy for the plant and organisms that consume the plant.
Elements used in large quantities by the plant are termed macronutrients, which can be further defined as primary or secondary. The primary nutrients include nitrogen (N), phosphorus (P), and potassium (K). These elements contribute to plant nutrient content, the function of plant enzymes and biochemical processes, and the integrity of plant cells. Deficiency of these nutrients contributes to reduced plant growth, health, and yield; thus they are the three most important nutrients supplied by fertilizers. The secondary nutrients include calcium (Ca), magnesium (Mg), and sulfur (S).
The final essential elements are used in small quantities by the plant, but nevertheless are necessary for plant survival. These micronutrients include iron (Fe), boron (B), copper (Cu), chlorine (Cl), Manganese (Mn), molybdenum (Mo), zinc (Zn), cobalt (Co), and nickel (Ni).
18 elements essential for plant nutrition, and classify the essential elements as macronutrients or micronutrients.
Macronutrients: used in large quantities by the plant
Structural nutrients: C, H, O
Primary nutrients: N, P, K
Secondary nutrients: Ca, Mg, S
Micronutrients: used in small quantities by the plant
Fe, B, Cu, Cl, Mn, Mo, Zn, Co, Ni
Nitrogen: found in chlorophyll, nucleic acids, and amino acids; component of protein and enzymes.
Phosphorus: an essential component of DNA, RNA, and phospholipids, which play critical roles in cell membranes; also plays a major role in the energy system (ATP) of plants.
Potassium: plays a major role in the metabolism of the plant, and is involved in photosynthesis, drought tolerance, improved winter hardiness, and protein synthesis.
Nitrogen availability limits the productivity of most cropping systems in the US. It is a component of chlorophyll, so when nitrogen is insufficient, leaves will take on a yellow (chlorotic) appearance down the middle of the leaf. New plant growth will be reduced as well and may appear red or red-brown. Because of its essential role in amino acids and proteins, deficient plants and grains will have low protein content. Nitrogen excess results in extremely dark green leaves, and promotes vegetative plant growth. This growth, particularly of grains, may exceed the plant's ability to hold itself upright, and increased lodging is observed. Nitrogen is mobile both in the soil and in the plant, which affects its application and management, as discussed later.
Phosphorus is another essential macronutrient whose deficiency is a major consideration in cropping systems. It is an essential part of the components of DNA and RNA, and is involved in cell membrane function and integrity. It is also a component of the ATP system, the "energy currency" of plants and animals. Phosphorus deficiency is seen as purple or reddish discolorations of plant leaves, and is accompanied by poor growth of the plant and roots, reduced yield and early fruit drop, and delayed maturity. Phosphorus excess can also present problems, though it is not as common. Excess P can induce a zinc deficiency through biochemical interactions. Phosphorus is generally immobile in the soil, which influences its application methods, and is somewhat mobile in plants.
Potassium is the third most commonly supplemented macronutrient. It has important functions in plant metabolism, is part of the regulation of water loss, and is necessary for adaptations to stress (such as drought and cold). Plants that are deficient in potassium may exhibit reductions in yield before any visible symptoms are noticed. These symptoms include yellowing of the margins and veins and crinkling or rolling of the leaves. An excess, meanwhile, will result in reduced plant uptake of magnesium, due to chemical interactions.
The mobility of a nutrient in the soil determines how much can be lost due to leaching or runoff.
The mobility of a nutrient in the plant determines where deficiency symptoms show up.
Nutrients that are mobile in the plant will move to new growth areas, so the deficiency symptoms will first show up in older leaves.
Nutrients that are not mobile in the plant will not move to new growth areas, so deficiency symptoms will first show up in the new growth.
Nutrient mobility varies among the essential elements and represents an important consideration when planning fertilizer applications. For instance, NO3- nitrogen is very mobile in the soil, and will leach easily. Excessive or improper application increases the risk of water contamination. Meanwhile, phosphorus is relatively immobile in the soil and is thus less likely to runoff. At the same time, it is also less available to plants, as it cannot "migrate" easily through the soil profile. Thus, P is often banded close to seeds to make sure it can be reached by starting roots. Nutrients also have variable degrees of mobility in the plant, which influences where deficiency symptoms appear. For nutrients like nitrogen, phosphorus, and potassium, which are mobile in the plant, deficiency symptoms will appear in older leaves. As new leaves develop, they will take the nutrients from the old leaves and use them to grow. The old leaves are then left without enough nutrients, and display the symptoms. The opposite is true of immobile nutrients like calcium; the new leaves will have symptoms first because they cannot take nutrients from the old leaves, and there is not enough in the soil for their needs.
In general, plant nutrient needs start low while the plants are young and small, increase rapidly through vegetative growth, and then decrease again around the time of reproductive development (i.e., silking and tasseling). While absolute nutrient requirements may be low for young plants, they often require or benefit from high levels in the soil around them. The nutrient status of the early seedlings will affect the overall plant development and yield. Plants entering the reproductive stages have high nutrient requirements, but many of these are satisfied by redistributing nutrients from the vegetative parts.
Nitrogen: nitrate (NO3-) and ammonium (NH4+)
Phosphorus: phosphate (HPO42- and H2PO4-)
Potassium: K+
Calcium: Ca2+
Magnesium: Mg2+
Sulfur: sulfate (SO4-)
4 likes
1 comment
Share
14
Week 14. Flowering1y ago
106.68 cm
Height
12 hrs
Light Schedule
27 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
23 °C
Night Air Temp
378.54 liters
Pot Size
30.48 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 6

RAW Phosphorus
2.6 mll

RAW Potassium
2.6 mll

Microbes Bloom Stage
1.3 mll
Ultraviolet Flowering Time 8 – 9 weeks
Potassium: A common deficiency in plants grown in sandy soils. Symptoms include yellowing, curling, and browning of leaves, as well as reduced growth and fertility.
Potassium is the third major component in fertilisers. Plants absorb Potassium as an ion, which can be readily leached and lost through run-off from the soil.
Potassium is needed by the plants to promote formation of sugars for protein synthesis, cell division in plants and for root development. It also increases the plant’s resistance to diseases.
Deficiency symptoms: Leaf edge chlorosis on new matured leaves followed by interveinal scorching and necrosis from leaf edge to the midrib as deficiency increases. The chlorosis in potassium deficiency is irreversible even if potassium is given to plants.
Nitrogen: A common deficiency that causes yellowing and stunted growth in plants. Nitrogen is easily washed out of the soil by winter rains, leaving plants deficient in spring. Remember Nitrates are highly mobile in soil and ammonium is highly immobile. Ammonium over 30% of total Nitrogen is a problem. 10-30% but no more.
Nitrogen is one of the major nutrients commonly applied as fertilisers. Plants absorb Nitrogen in the form of ammonium or nitrate which can be readily dissolved in water and leached away from soil.
Nitrogen is needed by plants to promote rapid growth especially for fruit and seed development. Also, it increases leaf size and quality, and hastens plant maturity.
Deficiency symptoms: General chlorosis of entire plant to a light green followed by yellowing of older leaves proceeding towards younger leaves. Plants become spindly, stunted and secondary shoots develop poorly if the initial symptoms are not corrected
Zinc: A deficiency that can occur in calcareous, high-pH soils that are sandy or have high soil-phosphorus levels. It's most common in spring when conditions are cool and wet.
Plants require zinc to activate plant growth regulators, particularly Auxin and Indole Acetic Acid (IAA).
Zinc is needed to activate plant growth regulators.
Deficiency symptoms: Chlorosis, bronzing or mottling of younger leaves. Interveinal chlorosis of the young leaves followed by reduced shoot growth with short internodes, as well as small and discoloured leaves giving the affected part a rosette appearance
Boron: A deficiency that can be caused by high or low pH, sandy soil with low organic matter, or lack of nitrogen.
Boron is absorbed from the soil by plants as borate.
Boron is needed in the process of cell differentiation at the growing tips of plants where cell division is active.
Deficiency symptoms: Plants become stunted and deformed. Proliferation of side shoots known as ‘witches broom’ can be observed as the main stem falls to ensure the growth of the lateral shoot stays dormat. This is known as the loss of apical dominance. In flowering shrubs, new growth becomes dark green and they develop cupped or puckered small brittle leaves with short internodes
Sulfur: A deficiency that can be caused by sandy soil with low organic matter or use of fertilizers that don't contain sulfur.
Iron: A deficiency that can be caused by high pH or soil low in organic matter.
Iron deficiency is similar to Magnesium, except that it appears on young leaves and shoots instead of older leaves.
Iron is needed by plants for the synthesis of chloroplast proteins and various enzymes.
Deficiency symptoms: Light green to yellow interveinal chlorosis on newly emerging leaves and young shoots. It is common to see shoots dying from the tip inwards. In severe cases, newly emerged leaves may reduce in size and turn nearly white, with necrotic
Phosphorus: A deficiency that can be caused by incorrect pH, nutrient imbalance, extreme cold, or excess iron in the growing medium.
Phosphorus is the second major component in fertilisers. Plants absorb Phosphorus in the form of phosphate.
Phosphorus is needed by plants to promote photosynthesis, protein formation, seed germination, bloom stimulation and budding. It also hastens maturity.
Deficiency symptoms: Purple or bronze colouration on the underside of older leaves due to the accumulation of the pigment, Anthocyanin. Affected plants develop very slowly and are stunted compared to normal plants
Calcium: A deficiency that can occur in acidic, alkali, or sodic soils.
Calcium is a constituent of plant cell wall and provides structural support to cell walls. It is immobile within plants and remains in the older tissue throughout the growing season. Hence first symptom of deficiency appears on the younger leaves and leaf tips.
Calcium is needed by plants to produce new growing points and root tips.
Deficiency symptoms: New foliage, buds and roots have stunted growth. Younger leaves curl downwards with browning of leaf edges and leaf tips, also known as tip burn. In some plants, they may also show abnormally green foliage. Roots become short and stubby.
Magnesium: A deficiency that occurs in similar conditions to calcium.
Magnesium is a structural component of the chlorophyll molecule.
Magnesium is needed by plants to promote the function of plant enzymes to produce carbohydrates, sugars and fats and in the regulation of nutrient absorption.
Deficiency symptoms: Older leaves are chlorotic in between veins, often known as interveinal chlorosis. In severe deficiency, plant growth rate drops, leaf size is reduced, and lower leaves are shed.
Manganese
Soil shortages are rare, but manganese and iron can be unavailable to plant roots in alkaline conditions. Ericaceous (acid-loving) plants are particularly vulnerable when growing in alkaline soils or potting composts.
Manganese acts as an enzyme activator for nitrogen assimilation.
Manganese is needed by plants for photosynthesis, respiration and enzyme reactions.
Deficiency symptoms: Newly emerging leaves exhibit a diffused interveinal chlorosis with poorly defined green areas around the veins. Chlorosis and necrotic spotting are common symptoms. In severe deficiency, new leaves become smaller and tip dieback can occur.
3 likes
comments
Share
15
Week 15. Flowering1y ago
106.68 cm
Height
12 hrs
Light Schedule
27 °C
Day Air Temp
6.5
pH
45 %
Air Humidity
23 °C
Substrate Temp
23 °C
Night Air Temp
378.54 liters
Pot Size
30.48 cm
Lamp Distance
1000 PPM
CO₂ Level
4 likes
comments
Share
16
Week 16. Flowering1y ago
106.68 cm
Height
12 hrs
Light Schedule
26 °C
Day Air Temp
6.2
pH
Weak
Smell
45 %
Air Humidity
20 °C
Substrate Temp
21 °C
Night Air Temp
378.54 liters
Pot Size
20.32 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 2
Ascorbic Acid
0.65 mll

RAW Calcium / Mag
3.91 mll
Ultraviolet (Vitamin C(Ascorbic Acid) is a coenzyme in the xanthophyll cycle, which converts excess energy into heat. This process helps plants protect themselves from too much light.
You can remove both chlorine and chloramine in water with the same strategies. Carbon filtration is a very effective method, but it takes a lot of carbon and water/carbon contact to do the job. That’s why Vitamin C (L-Ascorbic acid) is a better solution.
Does ascorbic acid/Vitamin C actually work to remove chlorine? Research by the Environmental Protection Agency (EPA) found that using ascorbic acid for chlorine is effective and works rapidly.
One gram of ascorbic acid will neutralize 1 milligram per liter of chlorine per 100 gallons of water. The reaction is very fast. The chemical reaction (Tikkanen and others 2001) of ascorbic acid with chlorine is shown below:
C5H5O5CH2OH + HOCL → C5H3O5CH2OH + HCl + H2O
Ascorbic acid + Hypochlorous acid → Dehydroascorbic acid + Hydrochloric acid + water
Vitamin C effectively neutralizes chlorine and is safer to handle than sulfur-based dechlorination chemicals. The sodium ascorbate form of vitamin C has less affect on pH than the ascorbic acid form. When neutralizing a strong chlorine solution, both forms of vitamin C will lower slightly the dissolved oxygen of the treated water.
5 likes
comments
Share
Used techniques
Defoliation
Technique
17
Week 17. Flowering1y ago
106.68 cm
Height
12 hrs
Light Schedule
28 °C
Day Air Temp
5.8
pH
Weak
Smell
45 %
Air Humidity
20 °C
Substrate Temp
20 °C
Night Air Temp
378.54 liters
Pot Size
20.32 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 4

RAW Calcium / Mag
0.65 mll

RAW Enzymes
0.33 mll

RAW Amino Acids
0.33 mll
Ultraviolet Gratitude. Gave her another application of Gibberelin, same as before.
What can I do to increase the rate of cellular respiration?
We are adding more reactants, like glucose.
Photosynthetic efficiency is the fraction of light energy converted into chemical energy during photosynthesis in green plants and algae. The simplified chemical reaction can describe photosynthesis
6 H2O + 6 CO2 + energy → C6H12O6 + 6 O2
where C6H12O6 is glucose (which is subsequently transformed into other sugars, starches, cellulose, lignin, and so forth). The value of the photosynthetic efficiency is dependent on how light energy is defined – it depends on whether we count only the light that is absorbed, and on what kind of light is used (see Photosynthetically active radiation). It takes eight (or perhaps ten or more) photons to use one molecule of CO2. The Gibbs free energy for converting a mole of CO2 to glucose is 114 kcal, whereas eight moles of photons of wavelength 600 nm contains 381 kcal, giving a nominal efficiency of 30%. However, photosynthesis can occur with light up to wavelength 720 nm so long as there is also light at wavelengths below 680 nm to keep Photosystem II operating (see Chlorophyll). Using longer wavelengths means less light energy is needed for the same number of photons and therefore for the same amount of photosynthesis. For actual sunlight, where only 45% of the light is in the photosynthetically active wavelength range, the theoretical maximum efficiency of solar energy conversion is approximately 11%. In actuality, however, plants do not absorb all incoming sunlight (due to reflection, respiration requirements of photosynthesis, and the need for optimal solar radiation levels) and do not convert all harvested energy into biomass, which results in a maximum overall photosynthetic efficiency of 3 to 6% of total solar radiation. If photosynthesis is inefficient, excess light energy must be dissipated to avoid damaging the photosynthetic apparatus. Energy can be dissipated as heat (non-photochemical quenching), or emitted as chlorophyll fluorescence.
Starting with the solar spectrum falling on a leaf,
47% lost due to photons outside the 400–700 nm active range (chlorophyll uses photons between 400 and 700 nm, extracting the energy of one 700 nm photon from each one)
30% of the in-band photons are lost due to incomplete absorption or photons hitting components other than chloroplasts
24% of the absorbed photon energy is lost due to degrading short wavelength photons to the 700 nm energy level
68% of the used energy is lost in conversion into d-glucose
35–45% of the glucose is consumed by the leaf in the processes of dark and photorespiration
Stated another way:
100% sunlight → non-bioavailable photons waste is 47%, leaving
53% (in the 400–700 nm range) → 30% of photons are lost due to incomplete absorption, leaving
37% (absorbed photon energy) → 24% is lost due to wavelength-mismatch degradation to 700 nm energy, leaving
28.2% (sunlight energy collected by chlorophyll) → 68% is lost in conversion of ATP and NADPH to d-glucose, leaving
9% (collected as sugar) → 35–40% of sugar is recycled/consumed by the leaf in dark and photo-respiration, leaving
5.4% net leaf efficiency.
Far-red
In efforts to increase photosynthetic efficiency, researchers have proposed extending the spectrum of light that is available for photosynthesis. One approach involves incorporating pigments like chlorophyll d and f, which are capable of absorbing far-red light, into the photosynthetic machinery of higher plants. Naturally present in certain cyanobacteria, these chlorophylls enable photosynthesis with far-red light that standard chlorophylls a and b cannot utilize. By adapting these pigments for use in higher plants, it is hoped that plants can be engineered to utilize a wider range of the light spectrum, potentially leading to increased growth rates and biomass production.
Green
Green light is considered the least efficient wavelength in the visible spectrum for photosynthesis and presents an opportunity for increased utilization. Chlorophyll c is a pigment found in marine algae with blue-green absorption and could be used to expand absorption in the green wavelengths in plants. Expression of the dinoflagellate CHLOROPHYLL C SYNTHASE gene in the plant Nicotiana benthamiana resulted in the heterologous production of chlorophyll c. This was the first successful introduction of a foreign chlorophyll molecule into a higher plant and is the first step towards bioengineering plants for improved photosynthetic performance across a variety of lighting conditions.
Photosynthesis by day,
Cellular respiration by night.
Notice that light intensity, carbon dioxide concentration, and temperature are the three main factors that impact photosynthesis. Greater light intensity leads to higher photosynthesis rates, as does increased carbon dioxide concentration. Temperature is also directly linked to the rate of respiration Q10 Temperature coefficient.
This is a key factor affecting photosynthesis. Low CO2 affects the Calvin Cycle. If CO2 levels are low, rubisco cannot convert RuBP to GP in step one of the Calvin Cycle. This leads to the accumulation of RuBP and an overall slowing of the Calvin Cycle, which results in a fall in the production of TP/GALP.
CO2 is not needed at night so turn it off. Nights should be focused dealing with excess moisture spat into the air all night long, ambient canopy RH 40-45%. This keeps a constant negative pressure overnight. Oxygen is what a plant needs at night, only oxygen diffuses into the leaves and only carbon dioxide diffuses out.
*in hindsight, I was oversaturating too long periods, the plant was gassing out.
4 likes
4 comments
Share
18
Week 18. Flowering1y ago
121.92 cm
Height
12 hrs
Light Schedule
28 °C
Day Air Temp
6.2
pH
Weak
Smell
45 %
Air Humidity
24 °C
Substrate Temp
20 °C
Night Air Temp
378.54 liters
Pot Size
15.24 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 8
Pure Honey
1.3 mll
Pure Organic Agave Nectar
5 mll
Carrot
1.3 mll
Ultraviolet Positively charged ions in plant nutrition are called cations:
Calcium: Ca+2
Magnesium: Mg+2
Potassium: K+
Sodium: Na+
Hydrogen: H+
Aluminum: Al3+
Iron: Fe2+
Manganese: Mn2+
Zinc: Zn2+
Copper: Cu2+
Cations are attracted to negatively charged soil particles, called the clay-humus-complex, because opposite charges attract. The soil's ability to hold onto cations is called its cation exchange capacity (CEC). Soils with a higher CEC can hold onto more nutrients.
The CEC of a soil depends on its clay and organic matter content. Sandy soils have a low CEC because they have larger particles and pore spaces, and a lower capacity to retain nutrients. Clay soils have a higher CEC because they have smaller particles and pore spaces, and clay particles have a higher surface area
Negatively charged nutrients in plant nutrition are called anions. Some common anions include: Phosphate (PO43-), Sulfate (SO42-), Nitrate (NO3-), and Chlorine (Cl-).
Anions are the opposite of cations, which are positively charged nutrients. The relationship between anions and cations in plants can cause significant changes in pH levels in the growing system. When plants absorb more anions than cations, the pH increases, and when they absorb more cations than anions, the pH decreases.
Here are some other things to know about anions:
Anions are held and retained by soil particles, but to a lesser extent than cations.
Anion adsorption is dependent on pH and increases as soil pH decreases.
Phosphates and sulfates are adsorbed more strongly than nitrates and chlorides.
Anions are more likely to be lost from the soil through leaching, with the exception of phosphate and sulfate to a lesser degree.
Over-application of a negatively charged element followed by excessive water will quickly move that element through the system
Among the micronutrients, Fe, Mn, Cu, Zn, and Ni are taken up by plants in their cationic forms, and B, Mo, and Cl are taken up by plants in their anionic forms.
3 likes
comments
Share
Used techniques
Defoliation
Technique
19
Week 19. Flowering1y ago
121.92 cm
Height
12 hrs
Light Schedule
24 °C
Day Air Temp
6.6
pH
Normal
Smell
45 %
Air Humidity
21 °C
Substrate Temp
14 °C
Night Air Temp
378.54 liters
Pot Size
15.24 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 4
Agave
5 mll

Bud Factor X
2 mll

RAW Cane Molasses
5.21 mll
Ultraviolet Most of the back ones fell over. I will look to cool things off now that trichome production is stepping up. Nope, they are all falling over.
Just destroying the distilled water intake,
Well, she is far from aesthetically pleasing but the goal was never bag appeal, at least not on this grow, this was balls to the wall with a slew of personal mini experiments to see what happens, how pretty/burnt the fan/sugar leaves look has no bearing on the quality of the smoke, that I know, 8-10 hours of UVB @ 4-500mW/cm2 for almost the entirety of the flower, acclimation, and tolerance built up with a full dose from early veg, the intense 1600-1800ppfd light intensity, way into 60moles on those highest colas. Isn't she supposed to have disintegrated 3 weeks ago with those exposure levels? What did I think would happen? I dunno, I wasn't thinking.
Evaporation
Evaporation is the transformation of liquid water into water vapour. It's a key process in the hydrological cycle, influencing climate and weather patterns globally.
Factors Affecting Evaporation Rates
Several factors impact how quickly evaporation occurs:
Temperature: Higher temperatures accelerate evaporation. As temperature increases, water molecules gain kinetic energy, making it easier for them to escape the liquid’s surface and become vapour.
Wind Speed: Wind removes moist air above a water body, facilitating further evaporation. Faster winds replace saturated air with drier air, enhancing evaporation rates.
Humidity: Low humidity levels increase evaporation. When the surrounding air is dry, it can absorb more water vapour, promoting evaporation from water surfaces.
Sunlight Exposure: Sunlight provides the energy necessary for evaporation. More sunlight equals more evaporation, as it heats the water and provides energy for water molecules to escape into the air.
Surface Area: A larger surface area allows more water to be exposed to air, hence increasing evaporation. Bodies of water with larger surface areas, like lakes, have higher evaporation rates than smaller bodies.
Energy Requirements
Evaporation is an endothermic process, meaning it absorbs heat. This heat, known as the latent heat of vaporisation, is necessary to break the molecular bonds of liquid water, allowing it to transition into a gas.
https://www.tutorchase.com/notes/cie-a-level/geography/2-3-1-atmospheric-moisture-processes
4 likes
comments
Share
20
Week 20. Flowering1y ago
121.92 cm
Height
12 hrs
Light Schedule
24 °C
Day Air Temp
6.6
pH
Strong
Smell
45 %
Air Humidity
21 °C
Substrate Temp
14 °C
Night Air Temp
378.54 liters
Pot Size
15.24 cm
Lamp Distance
1000 PPM
CO₂ Level
Nutrients 5
Agave Nectar
1.3 mll
Honey
1.3 mll

Bud Factor X
0.793 mll
Ultraviolet ....Understanding Electrical Conductivity
Electrical conductivity measures a material’s ability to transmit electric current, which in the context of gardening, relates to the soil or nutrient solution’s ion content. In simpler terms, EC indicates how salty the environment is around the roots of a plant. This “saltiness” is crucial because it affects the plant’s osmotic pressure, which in turn influences nutrient uptake and overall plant health.
The Science Behind EC
At its core, EC measures the presence of soluble ions like potassium, calcium, magnesium, and nitrates – all vital nutrients for plant growth. These ions carry electrical charges, and their movement creates an electrical current, detectable as conductivity. High EC levels typically mean a high concentration of dissolved ions, whereas low EC levels indicate fewer nutrients are available.
The Impact of Electrical Conductivity on Plant Growth
The relationship between EC and plant growth is a delicate balance. Just the right EC level can enhance nutrient uptake, bolster plant health, and increase yields. However, when EC levels stray too far from the optimal range, they can lead to nutrient imbalances, osmotic stress, and even plant death.
Nutrient Uptake and EC Levels
Plants absorb nutrients from the soil or water through their roots, a process influenced by the surrounding EC levels. Ideal EC levels help create an optimal environment for this exchange, ensuring plants receive the nutrients they need without exerting excessive energy.
High EC Levels: A Double-Edged Sword
While a certain level of dissolved ions is beneficial, excessively high EC levels can harm plants. High salinity can draw water out of plant cells, leading to dehydration and nutrient lockout – a condition where plants cannot absorb essential nutrients despite their presence.
Low EC Levels: Undernourished Gardens
Conversely, low EC levels can starve plants of necessary nutrients, stunt growth, and lead to underdeveloped or diseased plants. Maintaining an optimal EC range is crucial for healthy plant development.
Measuring and Adjusting Electrical Conductivity
Understanding the importance of EC is one thing, but applying this knowledge in the garden or greenhouse requires practical steps. Regular monitoring and adjustment can ensure that plants remain in a conducive growth environment.
Tools for Measuring EC
Gardeners can use various tools to measure EC, from simple handheld meters to more sophisticated systems integrated into hydroponic setups. Regular monitoring allows for timely adjustments to soil or nutrient solutions, ensuring optimal EC levels.
Adjusting EC for Optimal Growth
Adjusting EC involves changing the concentration of nutrients in the soil or solution. In hydroponics, this might mean diluting or concentrating the nutrient solution. In soil, amendments may be added or flushed with water to correct EC levels.
Practical Applications: EC in Various Growing Environments
The application of EC knowledge varies across different growing environments, from traditional soil-based gardens to modern hydroponic systems.
Soil Gardening
In soil, EC measurements can help diagnose nutrient imbalances and guide fertilization practices. Amending soil with organic matter or flushing with water can adjust EC levels to better support plant growth.
Hydroponics and Aquaponics
In hydroponic and aquaponic systems, where plants are grown in water-based solutions, maintaining optimal EC levels is crucial for nutrient availability. These systems allow for precise control of EC, directly influencing plant health and yield.
Greenhouse Cultivation
In greenhouses, EC monitoring can help manage the nutritional status of various plants, ensuring that each receives the right nutrient mix for optimal growth. Automated systems can provide real-time EC adjustments based on plant needs.
Navigating Challenges: Overcoming EC-Related Issues
While maintaining optimal EC levels can significantly enhance plant growth, gardeners may encounter challenges such as fluctuating conditions, equipment malfunctions, or environmental stresses. Regular monitoring, combined with a solid understanding of each plant’s specific needs, can mitigate these issues.
Conclusion: The Conductive Path to Lush Gardens
Electrical conductivity, though an invisible factor, is a cornerstone of successful gardening and farming. By understanding and managing EC levels, gardeners and farmers can profoundly influence the health and productivity of their plants. As we continue to explore the nuances of plant growth, the role of EC stands out as a testament to the marriage of science and nature in cultivating life. Whether in soil, water, or air, mastering the art of electrical conductivity can unlock the full potential of our green companions, leading to bountiful harvests and vibrant gardens.
5 likes
comments
Share
21
Week 21. Flowering1y ago
121.92 cm
Height
12 hrs
Light Schedule
20 °C
Day Air Temp
6.7
pH
Strong
Smell
40 %
Air Humidity
16 °C
Substrate Temp
12 °C
Night Air Temp
378.54 liters
Pot Size
30.48 cm
Lamp Distance
600 PPM
CO₂ Level
Ultraviolet Time to ripen in the cold.
5 likes
comments
Share
22
Week 22. Harvest1y ago
Happy Harvest Day!

8/10
Rated
The definition of a good hybrid right here is an eclectic mix. You'll instantly feel lifted with a positive sense that fills your brain with heady euphoria and a touch of creativity. This is accompanied by a boost of motivation that has you ready to focus on any task at hand, I found myself doing mundane tasks that I'd had on the back burner for months, odd little things. A relaxing body high accompanies this heady lift, keeping you kicked back although not sedated in the slightest.
It's hard to put a finger on what I'm smelling, just a strong dank smell, a mixture but nothing specifically comes through, creamy earth. little sour and peppery on exhale.
Impressive genetics again from Premium Cultivars, thank you, if this keeps up I might have my new seedbank.
Show more
Translate
Spent 142 days
Ger Veg Flo Har
1179 g
Bud wet weight per plant
335 g
Bud dry weight per plant
2
Plants
40.64 m²
Grow Room size
Normal
Difficulty

Euphoric, Relaxed, Creative
Positive effects

Sour, Cream, Earthy
Taste
Height
Day air temperature
Air humidity
PH
CO2
Light schedule
Night air temperature
Substrate temperature
Pot size
Lamp distance
Ultraviolet Day 3: 2358 grams net weight
7 likes
4 comments
Share
Equipment Reviews

the end.
Enjoying this diary? Follow for more updates!
Prefer the old Diary view?
Go back to the old Diary view