The Grow Awards 2026 🏆 



























"432" C#12

GrapeSweetsAuto
Soil
Soil
Indoor
Room Type
Topping
weeks 3
LST
weeks 5
Start at Harvest
G
Germination9mo ago
Ultraviolet A sound mind in a sound body is a short, but full description of a happy state in this World: he that has these two, has little more to wish for; and he that wants either of them, will be little the better for anything else.
Hello 👋. I've been really, really stoned for a month or two. I do enjoy a good cleaning. No need to rush this one, I've got a lot of stuff to tweak in the grow tent. Added a bunch of amendments to the medium and reloaded mineral content. Giving it a few weeks to let everything break down, settle in, and balance out before beginning, I'll be doing a thorough pH and EC prior to placing anything in the final pot and making sure to give it a good till in the top soil once everything is broken down into a more palatable size.
Added this grow.
500g Horticultural charcoal.
454g Elite Shungite Coal (Fullerene C60).
1kg Spirulina is approx NPK 10%, 100g/1kg (N), 20% 200g/1kg (P), and 20% 200g/1kg (K).
In 1 kg (1000g) of spirulina, you can expect approximately 50 mg to 177 mg of Zeaxanthin.
1 kg of moringa powder, approximate NPK : 4.8% N, 0.5% P, and 1.9% K.
500g Azomite. (Ultimate mineral micro nutrient).
1kg of Gaia green 4-4-4, 1kg of 4-4-4 fertilizer contains 4% which is equivalent to 40 grams of each in 1000g.
500g eggshells, 20grams of calcium per 500g.
Generally, the more organic matter a plant breaks down, the higher the soil's electrical conductivity (EC) tends to be. This is because the breakdown of organic matter releases nutrients into the soil solution, which increases the concentration of dissolved ions that conduct electricity. Roots and microorganisms do not directly compete for electrical conductivity itself. However, they do interact in the rhizosphere, a zone around plant roots where microbes and plants compete for resources like nutrients and water, which can indirectly influence electrical conductivity. Plant roots release exudates (carbon) into the soil, providing energy and nutrients for microbes. In return, microbes can help plants access nutrients, particularly in the rhizosphere, where nutrient availability can be high. Both roots and microbes compete for the same resources, such as phosphorus (P) and iron (Fe). Factors like microbial decomposition of plant-derived chelators and the proximity of microbes to the root surface can influence this competition.
The influence of water-soluble carboxylated light fullerene derivatives on the physiological plant state is not well studied.
The addition of Spirulina is primarily for zeaxanthin, as it contains a lot. But on top of that, Hawaiian Spirulina is cultivated in open ponds using a combination of 100% fresh potable water from Hawaiian aquifers and ultra-pure, deep ocean water containing all 94 trace minerals & elements. It is then gently dried using patented Ocean Chill Drying technology and cold-pressed to ensure maximum nutrient levels. Spirulina, a blue-green alga, is rich in nitrogen, phosphorus, and potassium (NPK), making it an excellent source of nutrients for plant growth. Studies have shown that Spirulina can be used as a biofertilizer, effectively replacing chemical fertilizers, especially for nitrogen with a whopping, NPK of 10% (N), 20% (P), and 20% (K).
Azomite needs no introduction; Just incase, Azomite delivers 70+ minerals and trace elements to boost BRIX, root strength, soil vitality, and yields. Micronized.
Moringa is a highly nutrient-dense plant, often called the "miracle tree" or "tree of life" due to its impressive nutritional profile and potential health benefits. It's particularly rich in vitamins, minerals, antioxidants, and other bioactive compounds, making it a valuable resource for addressing malnutrition and promoting overall health. Containing over 92 verifiable nutrients, Moringa oleifera is found to be the most nutrient-dense plant on earth. As far as we know this is the only place online where we list all 92 (and more) nutrients of the Moringa tree and we list all 46 antioxidants of the Moringa tree too. Deep in the Himalayas is a tree called Moringa oleifera, also known as the drumstick tree, and throughout the subtropics, this tree is cultivated for its amazing health benefits.
This amazing tree has the ability to grow in an array of conditions, and its health benefits are astonishing. The drumstick tree contains a staggering 92 nutrients and 46 natural antioxidants and as it also holds a number of anti-inflammatory compounds. The sheer number of nutrients found in this tree brings it to the top of the superfoods list, and in its native locations, it is said to have the ability to help treat more than 300 diseases and illnesses.
To give you an idea, just a single serving of the Moringa tree contains: 4 times the amount of calcium in the same amount of milk. More vitamin C than 7 oranges. Double the protein and 3 times the amount of potassium in a banana. It's high antioxidant levels can help fight free radicals, potentially slowing down the ageing process and promoting longevity. It is thought to be able to help lower cholesterol levels and regulate blood pressure, due to the high levels of niacin and vitamins B3 and B10.
A serving of 100g of this tree gives:
Over 8g protein
Over 400mg potassium
Nearly 450mg calcium
164mg vitamin C
738 ¼g of vitamin A.
Moringa also contains vitamins B1, B2, B3, B6, B7, A, C, K, E and D. Amino Acids: In addition, it contains various other nutrients, including the following essential amino acids:
Threonine – a nutrient that helps metabolism and prevents fatty buildup in the liver. It also aids digestion.
Isoleucine – good for a healthy brain and helps to give the body natural energy.
Leucine – works hand in hand with isoleucine to increase energy levels.
Phenylalanine – aids communication between the brain’s nerve cells and also helps to reduce hunger pangs as well as increasing alertness and improving memory.
Tryptophan – supports your immune system, and its mood boosting ability helps to beat depression and anxiety-associated insomnia. It also reduces the risk of heart attack and lowers bad cholesterol levels.
Lysine – aids the absorption of calcium into the bones, supports antibodies and regulates various hormones as well as inhibiting the growth of virus cells.
Methionine – provides the body with sulphur as well as helping to lower cholesterol. It also supports the liver, kidneys, and helps keep skin, hair and nails healthy.
Valine – helps to keep the mind calm.
There are other amino acids in Moringa too, which are not essential to the body but are still beneficial in supporting health. These include histidine, alanine, glutamic acid, arginine, cysteine, proline, aspartic acid, glycine, serine, and tyrosine. Moringa seeds contain oils that hold high levels of oleic acid, which may act to reduce inflammation.
https://amchara.com/detox-cleanse/92-nutrients-and-46-antioxidants-in-one-tree-maringa-oleifera/
4.8% N, 0.5% P, and 1.9% K. Moringa can also serve as a natural source of nitrogen, potentially replacing chemical fertilizers.
About 95% of the dry eggshell is calcium carbonate weighing 5.5 grams. The average eggshell contains about . 3% phosphorus and . 3% magnesium and traces of sodium, potassium, zinc, manganese, iron and copper.
1 ppm in water (mg/L) = 1 ppm in soil (mg/kg)
If you use 10 grams of 10-20-20 fertilizer in 4 liters of water:
Nitrogen: (10 grams * 10/100 * 1000000) / 4000 mL = 2500 ppm N
Phosphorus: (10 grams * 20/100 * 1000000) / 4000 mL = 5000 ppm P
Potassium: (10 grams * 20/100 * 1000000) / 4000 mL = 5000 ppm K
In summary, to determine the precise ppm of an NPK fertilizer, you need to know the specific weight of fertilizer used, the volume of water, and the NPK percentages. The general formula and example calculations can help you make these conversions.
12 likes
15 comments
Share
Used method
Directly In Substrate
Germination Method
1
Week 1. Vegetation9mo ago
Ultraviolet It is one thing to show a man that he is in an error, and another to put him in possession of the truth.
Light acts as the pivotal external environment cue to modulate plant growth and development. Seeds germinated in the soil without light initially undergo skotomorphogenesis with rapidly elongating hypocotyls that facilitate emergence from the soil, while seedlings germinated using light exposure undergo photomorphogenesis with significantly inhibited hypocotyl elongation that benefits plants to stand up firmly and cope with the changing environment. Light promotes jasmonate (JA) biosynthesis to inhibit hypocotyl elongation and orchestrate seedling photomorphogenesis, now you know.
In normally aerated soil, plants grow “soil roots” to absorb water and oxygen from the soil. When the roots are put in water or waterlogged soil, the soil roots will rot due to a lack of oxygen and will be replaced by “water roots” that have special mechanisms to breathe through the leaves and block oxygen loss. In regular soil, there are air-filled pores in between soil particles. When the soil is waterlogged or saturated with water, the pores are filled with water molecules instead of air, thus creating a low or no-oxygen environment for soil roots. When plant roots are submerged in water or waterlogged soil, or even when there is a film of water on roots, “soil roots” will die off and will be replaced by “water roots”.
Water roots have two special features that allow them to survive in a low- or no-oxygen environment. Water roots have extensive aerenchyma plant tissues to enable the roots to continue to breathe through the leaves in a low-oxygen or waterlogged environment. The cells in the root area need oxygen to survive. When plant roots are put under water or in waterlogged soil, the root cells cannot receive enough oxygen from the surroundings and will rot and die off. The hormone ethylene is then released and triggers the formation of aerenchyma in the new roots (water roots). Aerenchyma are hollow air channels inside a root that connect to the stems and leaves, allowing the root cells to continue getting oxygen from the shoots above water. Water roots have extensive aerenchyma structures. Because of the hollow structure of aerenchyma filled with gas, water roots are thicker with a bigger diameter, more spongy, more brittle, and easier to break than soil roots. Apart from that, water roots also look more white and elongated than soil roots. Depending on the plant species, most soil roots also have aerenchyma but at a much lower density than water roots, and would thus die in a low-oxygen environment because of suffocation.
Another special feature water roots have to adapt to a low-oxygen environment is to develop a wax coating on the water roots. This coating is made of a waxy substance called “suberin” which acts as a barrier that prevents the entry and loss of oxygen, water, salts, and toxic compounds into and from the roots. The more difficult the environment (e.g. low oxygen, high salinity, a high number of pathogens), the more this waxy coating covers the whole length of a root, except the root tip. In contrast, soil roots do not have such a waxy barrier to the surrounding environment. With a pore size of 3.5 to 5.2 nm in diameter, water, oxygen, and salts can move in and out freely, causing soil roots to rot and die in a low-oxygen environment. Also, since pathogens can freely enter porous soil roots, that explains why soil roots are more susceptible to pathogen attack and rot in a waterlogged environment.
Another reason why soil roots rot in waterlogged soil is that there are more pathogens in soil than in water. Many soil-borne pathogens, including fungi (e.g. Pythium, Phytophthora) and bacteria (e.g. Oomycetes, Rhizoctonia solani) can survive in the soil even after the use of the herbicide. Also, when we grow plants in a water culture, we tend to change the water much more often than we change the soil, and the tap water we use most likely contains chlorine.
The concentration of oxygen in water is higher than in soil that is saturated with water. Water culture generally contains more oxygen than waterlogged soil because it is often changed from time to time. Also, oxygen moves about 10,000 times slower in water than it does in the air, but moves much more slowly (320,000 times less than in air) when the soil pores are filled with water (Armstrong & Drew, 2002). Also, there is a high population of microorganisms in the soil that compete for oxygen, quickly turning the wet soil anaerobic, which can easily suffocate soil roots.
Cannabis typically develops soil roots, which are also known as taproots and fibrous roots. These roots are primarily designed to anchor the plant in the soil and absorb nutrients and water. While some plants can root in water, cannabis, like most land plants, is adapted to grow with its roots in soil.
While cannabis can be grown in hydroponic systems (using water instead of soil), the roots in these systems will be different from soil roots. Water roots are typically thinner and more delicate, with more hairs to absorb oxygen from the water.
Cannabis plants can develop both soil roots and water roots. Soil roots are typically thicker and more robust, while water roots (also known as aerial roots) are finer and more delicate, with more root hairs. The development of water roots can be observed in plants like orchids, where they help absorb moisture from the air or on the plant's surface. Cannabis water roots can be successfully transitioned to soil roots, but it's a gradual process that requires careful attention. While water roots are designed for absorbing oxygen from water, they can adapt to a soil environment by making sure to provide enough oxygen to ease transition. Oversaturated soil below O2 thresholds will kill water roots in normal "above soil" RH% conditions.
10 likes
1 comment
Share
2
Week 2. Vegetation9mo ago
18 hrs
Light Schedule
30 °C
Day Air Temp
6.7
pH
650 PPM
TDS
45 %
Air Humidity
Ultraviolet I have always thought the actions of men the best interpreters of their thoughts.
Seedling managing 93F 30%RH, around 20 DLI. Vpd is in the 3's. No I don't recommend.
Germination date is May 22nd, 80 days auto would be August 10.
Color change from 24th to 25th, that's the roots penetrating the recycled paper pot and into the high-carbon soil.
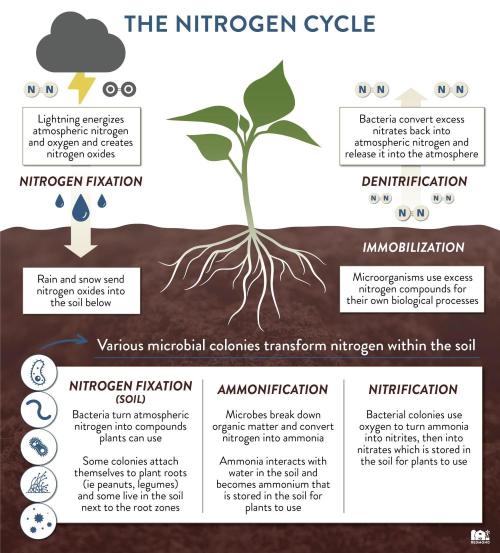
The plant nutrient nitrogen exists in forms with both positive and negative charges. Ammonium (NH4+)(immobile in soil)(Cation) has a positive charge, while nitrate (NO3-) (highly mobile in soil)(Anion)has a negative charge. Nitrogen is unique among plant nutrients in that it can exist in both positively charged (ammonium, NH₄⁺) and negatively charged (nitrate, NO₃⁻) forms in the soil. This makes it a special nutrient. In that it is responsible for providing balance for reactionary trade offs when it comes to ph. Because ph itself in the medium will always slowly drift towards acidicity, such is nature. 80% of nitrogen should be nitrate and no more than 20% ammoniacal nitrogen.
Ca, mg, and K are the big 3 cations related to soil composition, pH & base saturation.
When nitrogen is in the form of ammonium, it can compete with calcium, magnesium, and potassium for absorption sites in the plant root. This competition can lead to a reduction in the uptake of these other essential nutrients. Nitrogen, particularly in its nitrate form (NO3-), can increase soil acidity, which can also affect the availability of calcium, magnesium, and potassium. The form of nitrogen applied (ammonium vs. nitrate) can influence its interactions with other nutrients. Ammonium nitrogen can have a more pronounced negative effect on the uptake of calcium, magnesium, and potassium compared to nitrate nitrogen.
Nitrate (NO3-):
This is the form of nitrogen most easily absorbed by plants. It's also the most mobile in the soil, meaning it can be easily leached away by water.
Ammonium (NH4+):
This form of nitrogen is less mobile and is held more tightly by soil particles. It needs to be converted to nitrate by soil bacteria before plants can readily use it. Factors like soil temperature, moisture, and pH influence the conversion of ammonium to nitrate and the overall availability of nitrogen.
The ideal pH for the conversion of ammonium (NH4+) to nitrate (NO3-) through nitrification is typically between 6.5 and 9.0, with the optimal range being above 7.5 and below 8.5. This process is carried out by bacteria and is sensitive to pH levels, with acidic conditions being particularly unfavorable.
nitrification, the process of converting ammonium (NH4+) to nitrate (NO3-), typically leads to a decrease in pH. This is because the process releases hydrogen ions (H+), which increase the acidity of the solution and lower the pH.
Common forms of ammonium nitrogen include ammonium ion (NH4+), urea, and ammonium compounds like ammonium nitrate, ammonium sulfate, and ammonium phosphate.
Common forms of nitrate nitrogen include potassium nitrate (KNO3), sodium nitrate (NaNO3), calcium nitrate (Ca(NO3)2), and ammonium nitrate (NH4NO3).
Phosphorus & Oxygen.
Phosphorus is an essential plant nutrient, and its availability in the soil is strongly linked to the presence of oxygen. Plants primarily absorb phosphorus as phosphate (PO4), and oxygen is a key component of this molecule. Furthermore, the availability of phosphorus in the soil can be impacted by factors like soil aeration and temperature, which in turn affect the oxygen supply to the roots. Phosphorus uptake in plants is most critical during the early stages of growth, particularly within the first few weeks of plant development. Young plants actively growing tissues have a high demand for phosphorus. They may absorb up to 75% of their total phosphorus requirements within the first few weeks of vegetative growth, with up to 51% of uptake happening overnight, primarily in the first few hours of nightfall. (Controlled comditions) but worth noting.
Anaerobic root respiration, or respiration without oxygen, is detrimental to plants because it's less efficient and produces toxic byproducts, leading to reduced energy production, nutrient uptake issues, and ultimately, root damage and plant stress. Anaerobic respiration, unlike aerobic respiration, doesn't utilize oxygen as the final electron acceptor in the electron transport chain. This results in a significant drop in the amount of energy (ATP) produced, which is necessary for various plant functions, including growth, nutrient uptake, and maintenance of cellular processes. In the absence of oxygen, plants produce byproducts like ethanol and lactic acid during anaerobic fermentation. These byproducts can be toxic to the roots and inhibit their function, When oxygen is depleted in a medium, the pH tends to decrease (become more acidic) due to the production of metabolic byproducts. This is particularly relevant in biological systems where aerobic respiration relies on oxygen as the final electron acceptor. When oxygen is scarce, plants may switch to anaerobic respiration. This process produces carbon dioxide (CO2) as a byproduct.CO2 dissolves in water to form carbonic acid (H2CO3). This acid lowers the pH of the medium, making it more acidic. Anaerobic conditions can impair a plant's ability to regulate its internal pH, leading to a drop in cytoplasmic pH and potentially cellular acidosis. The change in pH can also affect the availability of certain nutrients to the plant, as pH influences the solubility of micronutrients like iron, manganese, zinc, copper, and boron.
The lack of oxygen in the plant medium leads to a decrease in pH due to the production of carbon dioxide during anaerobic respiration and impaired pH regulation within the plant. In plant cells, cellular acidosis, a drop in the internal pH of the cytosol, is a significant stress response, particularly during conditions like flooding or hypoxia. This acidification can be triggered by a decrease in oxygen levels, leading to the production of metabolic byproducts like lactic acid and CO2. The plant's ability to tolerate and recover from these conditions depends on its cellular mechanisms to regulate pH and mitigate the effects of acidosis. When plants are subjected to low oxygen environments, such as those experienced during flooding, anaerobic metabolism, which produces lactic acid and ethanol, becomes the primary source of energy. This can lead to a build-up of these acidic metabolites in the cytosol, causing a drop in pH. Critical to understand if you ask me.
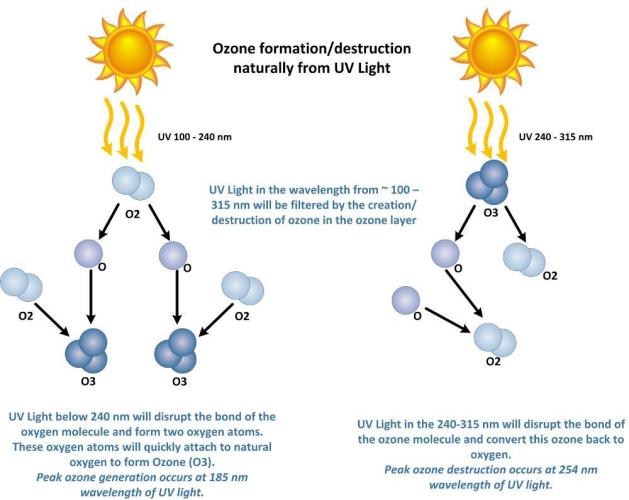
Oxygen & Ozone, and its relation to Ultraviolet light.
Atomic oxygen (single oxygen atom, O) is the lightest form of oxygen, as it has the lowest mass of the oxygen molecules. Oxygen also exists as a diatomic molecule (O2) and an allotrope called ozone (O3), which have higher masses due to the number of oxygen atoms combined.
Atomic Oxygen (O):
This refers to a single oxygen atom, which is the most fundamental form of oxygen.
Molecular Oxygen (O2):
This is the common form of oxygen we breathe, consisting of two oxygen atoms bonded together.
Ozone (O3):
This is an allotrope of oxygen, meaning it's a different form of the same element, consisting of three oxygen atoms bonded together.
Since atomic oxygen has the fewest oxygen atoms, it naturally has the lowest mass compared to O2 or O3.
Ozone (O3) Lifespan:
Ozone has a relatively long lifespan in the stratosphere, particularly at lower altitudes. For example, at 32 km in the middle latitudes during spring, ozone has a lifetime of about 2 months.
Oxygen (O) Lifespan:
Atomic oxygen, on the other hand, has a much shorter lifespan. At the same altitude, its lifetime is about 4/100ths of a second.
Ozone-Oxygen Cycle:
The ozone-oxygen cycle involves the rapid exchange between atomic oxygen (O) and ozone (O3). UV radiation can split molecular oxygen (O2) into atomic oxygen (O), which then reacts with O2 to form ozone (O3). Ozone can also be photolyzed by UV radiation, creating atomic oxygen again, which can then react with O3 to reform O2.
Dominant Form:
The partitioning of odd oxygen (Ox) between ozone and atomic oxygen favors ozone in the lower stratosphere. This means that a much larger proportion of odd oxygen exists as ozone than as atomic oxygen, especially in the lower stratosphere.
Recombination:
Atomic oxygen has a high energy and reactivity. When it encounters another oxygen atom, they can combine to form O2. This process releases energy, contributing to the heating of the atmosphere.
Ozone Formation:
Atomic oxygen can also react with molecular oxygen (O2) to form ozone (O3). Ozone plays a significant role in absorbing harmful UV radiation.
Other Reactions:
Atomic oxygen can react with various other molecules in the atmosphere, like nitrogen (N2), water (H2O), and carbon dioxide (CO2), forming different compounds.
UV light below 240nm (peak 185nm) creates ozone (O₃) through a process called photolysis, where UV light breaks down dioxygen molecules (O₂) into single atomic oxygen atoms (O). These single oxygen atoms then react with other oxygen molecules to form ozone (O₃). Specifically, UV-C light with wavelengths shorter than 240 nm can cause this photolysis.
UV light with wavelengths between 240-280 nm, (peak 254 nm) breaks down ozone (O₃) into dioxygen molecules (O₂) and atomic oxygen atoms (O). 280nm does not have the energy potential to break apart the stable bond of (O₂) into enough (O) to make (O₃)
At ground level, atomic oxygen (single oxygen atoms) has a very short lifespan. This is because it's highly reactive and quickly combines with other molecules to form stable diatomic oxygen (O2) or other compounds. While the exact timeframe varies depending on the specific circumstances, its lifespan is typically measured in nanoseconds or picoseconds.
9 likes
comments
Share
3
Week 3. Vegetation8mo ago
12 hrs
Light Schedule
29 °C
Day Air Temp
6.7
pH
650 PPM
TDS
35 %
Air Humidity
Ultraviolet You can dose a plant 40DLI, and it will grow. DLI is a measure of the potential energy captured in one cycle. Turgor pressure dictates cell elongation and growth. Turgor is dictated (daytime 90%) by transpiration. During transpiration, the plant pulls water from the roots, which creates osmosis. It is this osmosis that creates the pressure the plant uses to stretch cell membranes and grow rapidly. Turgor pressure is the counter-pressure the roots use to penetrate the soil. Osmosis plays a vital role in plants' nutrient uptake, particularly in the initial stages of absorption from the soil. Water, along with dissolved nutrients, moves into root cells via osmosis due to a concentration gradient. This process is driven by the higher solute concentration inside the root cells compared to the surrounding soil water, causing water to flow in the direction of higher salinity. Increased transpiration generally leads to increased nutrient uptake in plants. Transpiration, the process of water movement through a plant and evaporation from its leaves, creates a pulling force (cohesion-tension) that draws water and dissolved nutrients up from the roots. This "mass flow" of water brings nutrients into the plant. However, if transpiration is too high or too low, it can negatively impact nutrient uptake. While increased transpiration can lead to increased nutrient uptake, there is an optimal range. If transpiration is too low (water stress), nutrient uptake can be limited. If transpiration is too high, plants may close their stomata to reduce water loss, which can also limit nutrient uptake.Some nutrients, like calcium, are passively taken up by the plant, meaning they move along a concentration gradient (from an area of high concentration to an area of low concentration). This passive uptake is driven by transpiration. Other nutrients may require active transport, where the plant uses energy to move them across the cell membrane.
Normal drinking water generally contains 5-7 mg/L of dissolved oxygen. Fresh fountain water can have slightly higher levels, around 10-12 mg/L. Commercially produced oxygenated waters are designed to have a significantly higher concentration of dissolved oxygen, ranging from 30 to 120 mg/L. Some commercially available hyperoxygenated waters can reach levels of 80 ml O2/L (STPD).
Apologies for rambling on, but it's important to remember how important oxygen is to everything; if it runs short in supply for a moment, anaerobic respiration takes over, lactic acid, ATP drops, growth hinders, and pH skews. Nutrients are the construction materials. Light is the energy. Water is the solvent used to transport these minerals. Osmosis builds the pressure, and the higher the pressure, the higher the rate of nutrient cycling, given that conditions are optimized. Microorganisms will compete with the plant's root zones for oxygen. Microbial activity in soil can be more pronounced during flowering than during the vegetative stage of a plant's life. This is because flowering involves much increased nutrient demands and changes in plant exudates, which can stimulate microbial activity in the rhizosphere. This is coupled with soil becoming slightly more moisture-retaining as more and more exudates are fed to the micros. Basically they go into overdrive, and that means they scavenge much higher levels of oxygen. less oxygen for the essential plant processes.
High light intensities can lead to increased leaf thickness, density, and leaf dry mass, but also more stomata per mm2. Stomata are pores in the leaf that help with gas exchange (CO2 intake for photosynthesis) and water vapor release (transpiration). In essence, a high DLI (40) provides the light energy for photosynthesis, but the lack of water movement will severely hinder the plant's ability to utilize that energy and thrive. The plant will likely experience stress, wilting, and stunted growth.
C60, also known as fullerene C60, has been shown to inhibit the production of Reactive Oxygen Species (ROS). This is often attributed to its antioxidant properties and its ability to interact with mitochondria, leading to a mild uncoupling of respiration and phosphorylation, thus decreasing ROS production. This grow, I'll mostly be doing a slew of high-intensity testing, I want to observe for myself, I'm selfish like that.
Inhibit by decreasing ROS production, you say. How does that change the mechanics of it? Meaning the soft cap on how much light can be utilized is lifted? I need to observe for myself under extreme duress.
Plants, Reactive Oxygen Species (ROS) are primarily produced during photosynthesis and respiration. When a plant experiences excess light, it can produce reactive oxygen species (ROS), which can be harmful to the plant. While cannabis plants have mechanisms to deal with ROS, the increased production under excess light can overwhelm these defenses and cause damage. In these situations, the plant's ability to photosynthesize efficiently may be limited due to the damage caused by excessive ROS. Xanthophyll Cycle, NPQ, ROS & Zeanthaxin are key to better understanding the underlying mechanism and how plants deal with excess light in extreme intensities. Plants utilize non-photochemical quenching (NPQ) as a crucial mechanism to dissipate excess energy and prevent photo-oxidative damage caused by reactive oxygen species (ROS). The xanthophyll cycle and Non-photochemical Quenching (NPQ) are tightly linked mechanisms that help plants dissipate excess light energy to prevent damage to the photosynthetic apparatus. The xanthophyll cycle, involving the conversion of violaxanthin to zeaxanthin, plays a key role in the induction and regulation of NPQ, a process where plants convert excess energy into heat. The conversion of violaxanthin to zeaxanthin is a crucial part of the xanthophyll cycle, a photoprotective mechanism in plants. This cycle is triggered by light stress, including UV-A radiation. This is why it's important to me in sunset and sunrise, to mimic nature; this helps protect the photosynthetic apparatus from damage. Violaxanthin de-epoxidase (VDE) catalyzes the conversion of violaxanthin to zeaxanthin, with antheraxanthin as an intermediate, under conditions of high light stress. Master Key. UV-B @ solar noon with extra 450nm to 495nm to drive the photolyase DNA repair mechanism, repairing 100% of cellular dmg done from high intensity UV-B, Organisms have enzymatic antioxidant systems like superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) that help scavenge ROS and convert them into less harmful molecules. Non-enzymatic antioxidants, such as N-acetyl-l-cysteine (NAC) and ascorbic acid, also play a role in scavenging ROS. I will facilitate. She will grow. Mr Crow told me so.
Will the plant just stress into weirdness as its production of ROS is being "inhibited" to help deal with the rigours of excessively high light intensities? Still early days, but I was not expecting a seedling to be able to handle 93°F 30RH% even if it was only for a day or two. That puts VPD in the 3s, which is just insane for a seedling. Would have preferred photoperiods for this one, but we will make do with autos, don't like being on a timer from day 1, I wanted to take my time and mess around in veg. For now, we can see how it goes, I guess, and if it messes up, I'll just blame bad genetics. If it goes well, then it's all in a day's work! If the rate of nutrient uptake exceeds the plant's ability to utilize them or if the soil nutrient supply is imbalanced, the plant can accumulate toxic levels of certain nutrients within plant tissue. Soooo, in that case, I'll just create a bigger demand, lift the cap to see how hard big and beautiful we can plump her up!
You can put all the gasoline in a car you want, but if the mechanism that drives is not in high gear, you cannot travel far in a cycle.
You can put all the DLI you want on a plant; if it's not uptaking efficiently with a lot of turgor pressure built up, it will not be able to "stretch" and grow much in a cycle.
If you crank the transpiration 2x its regular speed, there will be a need for 2x the water. For double the growth, you will need double the mineral salt electrolytes in order to facilitate that growth.
Salt left to evaporate in "water" will crystallize. When water evaporates, the dissolved salt molecules become more concentrated and, as the water continues to evaporate, they can no longer remain dissolved. This causes them to come out of the solution and form solid crystals. Think "water in medium" Interestingly with everything I've been reading on base saturation and how important Ca, Mg, and K are to soil composition, gets my mind noticing, because ive added so much cations, I observed how much more structured the soil becomes after a heavy top watering, as the top soil evaporates, the soil almost looks as if it forms a structure due to high level of salts in the medium susceptible to the effect of rapid evaporation daily, obviously I'm not talking about perfect structure, its soil, dirt, but its just so airy, you can just see how much oxygen could easily penetrate, sorta adds a little internal salt-like crystal skeleton to the soil to help give it structure, crumbles under any pressure, this cycle of evaporation creates porous pockets of air rather than a flat dense muck. Just something I noticed. Really nice soil for growth.
Topped all 4 plants on day 10. She will need to grow into the high intensity. At current height, can't get it a over 500 ppfd.
8 likes
comments
Share
Used techniques
Topping
Technique
4
Week 4. Vegetation8mo ago
12 hrs
Light Schedule
29 °C
Day Air Temp
45 %
Air Humidity
Nutrients 2

RAW Enzymes
0.33 mll

RAW Amino Acids
0.33 mll
Ultraviolet The dread of evil is a much more forcible principle of human actions than the prospect of good.
High temperatures, high transpiration, high turgor pressure, and lots of cell elongation. Cannabis plants can sense and respond to their surroundings. They don't have brains like humans, but they have evolved sophisticated ways to perceive and react to various environmental cues, including light, gravity, touch, water, sound, and even the presence of other plants. This ability, known as plant perception, allows them to adjust their growth, defend themselves, and even communicate with each other.
Infrared light, particularly near-infrared (NIR), can influence plant growth and development by impacting various processes including initiation and elongation.
UVB radiation inhibits stem and hypocotyl elongation in plants.
Moderate UV-B exposure can cause a decrease in stem and leaf elongation, leading to a more compact plant architecture. Higher UV-B intensities, like 7.5 kJ·m−2, can lead to reduced elongation.
So it becomes a dance of initiating a stretch to seek to higher ppfd, then whenever you want the stretching to slow down,UVB inhibits the stretch by altering hormone balance,, coupled with the high-intensity dli once you get her to a height where you want. Being autos I don't have much time to play around. So we are playing fast and loose, get stretched, and then spread her open. You get the idea.
86F 30% RH with the lower side of optimal on ppfd, with a smudgering of infra-red to initiate response of making her stretch more so than normal.
Once she sprouts up in height, I drop metrics to 77F 55%RH% while upping the ppfd, giving her a dash of extra 430nm, giving the grow more of a 6000K. This encourages denser growth with less elongation. Higher side of optimal PPFD, with the auxin hormone balance changed, the side stems are starting to shoot up to the same canopy level as the apex (almost). Normally takes me 2 untrained toppings before a plant will flatten its canopy. I didn't take enough off the apex to do it in one chop, it was only day 10, so there really wasn't much to top from without creating unpredictable results using fim, opted just to keep it to a clean topping. Even predictability over the Chaos of the"Fim".
Brainfart:
Remember, the veg stage creates the skeleton that will stretch out into flowers. How dense you create the skeleton through light intensity will determine the density of your clusters as they form cola, as long as you can keep up with the demands. Increasing light intensity generally leads to plants having shorter internodal spacing, meaning the distance between nodes (where leaves grow) on a stem is reduced. This effect is particularly noticeable under LED grow lights, with great stress comes great dense clusters. Hello, temperature, my old friend! Goodbye, money on AC.
What did the flower decide to study in college?
STEM.
11 likes
2 comments
Share
5
Week 5. Vegetation8mo ago
12 hrs
Light Schedule
25 °C
Day Air Temp
50 %
Air Humidity
24 °C
Night Air Temp
Nutrients 4

RAW Grow
0.65 mll

RAW B-vitamin
0.33 mll

RAW Bloom
0.65 mll
Ultraviolet Reverie is when ideas float in our mind without reflection or regard of the understanding.
Managed to break a main stem, tried my best to get her back on in rapid fashion, but it was a 95% clean break, so I can't expect 🙃 much. Oh well, that's what I get for cracking bad jokes..
https://onlinelibrary.wiley.com/doi/full/10.1111/pce.12934
Photomorphogenic responses to ultraviolet-B light
Gareth I. Jenkins
First published: 09 February 2017
https://doi.org/10.1111/pce.12934
Citations: 173
Interestingly, evidence is emerging of a role for UVR8 in plant defence. UV-B exposure of plants increases resistance to attack by various pests and pathogens (Roberts & Paul 2006; Ballaré et al. 2012), and this is principally due to biochemical changes, in particular the synthesis of inhibitory or unpalatable compounds. UVR8 action contributes to some of these protective responses. Morales et al. (2013) found that UVR8 mediates the UV-B-induced expression of several genes concerned with countering attack by herbivorous pests in Arabidopsis. In addition, the ability of UV-B exposure to reduce infection by the fungus Botrytis cineria is diminished in uvr8 mutant plants (Demkura & Ballaré 2012). This protection is likely due to UVR8-induced synthesis of sinapic acid derivatives, because the protective response is also absent in plants defective in sinapate biosynthesis. A recent report suggests that UVR8 may be involved in systemic acquired resistance (SAR) in Arabidopsis (Carella et al. 2016). The abundance of UVR8 in phloem exudates of leaves decreased following infection with strains of Pseudomonas syringae that induce SAR, relative to controls. Both uvr8 mutant and UVR8 over-expression lines showed reduced SAR compared to wild type, and the authors suggested that UVR8 might have distinct positive and negative regulatory roles in SAR. However, the experiments were reportedly undertaken in light conditions lacking UV-B, so it is not clear how UVR8 might be acting in this response.
A recurrent theme in recent research is that UVR8 often functions through interaction with other signaling pathways. In particular, several studies highlight an interaction between UVR8 and the hormonal pathways that regulate extension growth. One example is the role of UVR8 in suppressing the shade avoidance response. Many plant species respond to the presence of neighbouring vegetation by stimulating extension growth as a result of increased auxin biosynthesis. Leaves absorb red light but reflect far-red light, and therefore shading by vegetation leads to a relative decrease in the ratio of ambient red:far-red light, which is detected by phytochrome, causing a decrease in Pfr relative to Pr (Casal 2013; Fraser et al. 2016). In turn, the decrease in Pfr/Pr leads to an increase in stability and activity of several PHYTOCHROME INTERACTING FACTOR (PIF) transcription factors, notably PIFs 4, 5 and 7, which stimulate expression of auxin biosynthesis genes, leading to extension growth (Hornitschek et al. 2012; Li et al. 2012). Hayes et al. (2014) showed that UV-B antagonizes shade avoidance responses in Arabidopsis elicited by low red:far-red light, and the UV-B effect was strongly impaired in uvr8 mutant plants. UV-B, detected by UVR8, inhibited the increase in expression of auxin biosynthesis and signaling genes promoted by reduced red:far-red light. Furthermore, UVR8 signaling stimulated GA2OXIDASE1 expression, which causes reduced levels of gibberellic acid and consequent stabilization of DELLA proteins, which antagonize PIF activity (De Lucas et al. 2008; Feng et al. 2008). Whereas the effect of UV-B on GA2OXIDASE1 expression required HY5/HYH, that on the auxin related genes did not. The experiments further showed that UV-B elicited destruction of PIFs 4 and 5 and the stabilization of DELLA proteins, although it remains to be established directly whether the effects on these proteins are mediated by UVR8. Thus, UV-B, detected by UVR8, signals to plants that they are in sunlight and negates shade-induced extension growth by antagonizing PIF action and auxin biosynthesis.
UV-B also inhibits the morphogenic responses caused by exposure to elevated temperature, which include hypocotyl extension in seedlings and petiole extension and leaf elevation in mature plants; again, the effect of UV-B is substantially mediated by UVR8 (Hayes et al. 2016). However, in contrast to the action of UV-B in suppressing shade avoidance, UV-B inhibition of thermomorphogenesis does not involve either PIF destruction or an effect on DELLA proteins. PIF4 is a key regulator of thermomorphogenesis, promoting expression of genes concerned with auxin biosynthesis and signaling. UV-B inhibits PIF4 transcript accumulation, consequently preventing an increase in PIF4 protein, and also stabilizes the LONG HYPOCOTYL IN FAR-RED 1 transcription factor, which binds to PIF4, impairing its ability to bind to DNA. Together, these mechanisms block the accumulation and activity of PIF4 at elevated temperature (Hayes et al. 2016). The inhibition of thermomorphogenesis by UV-B is likely to be advantageous for plants, as it will prevent detrimental extension growth under natural conditions where elevated temperature is often accompanied by exposure to relatively high levels of UV-B.
Another auxin-regulated growth response is phototropism. It is well established that phototropism in response to unilateral UV-A/blue light is mediated by phototropins, which direct accumulation of auxin on the non-illuminated side of the stem, causing localized extension and hence bending towards the light source (Christie & Murphy 2013). Vandenbussche et al. (2014) reported that UV-B can also induce phototropic bending and that the UV-B response in phot1phot2 mutant plants requires UVR8. However, UV-B-induced bending is slower in phot1phot2 than in wild type, indicating that phototropin action is involved in the wild-type UV-B response, and that the phototropin-mediated response is faster than that mediated by UVR8 (Vandenbussche & Van Der Straeten 2014; Vandenbussche et al. 2014). Moreover, the response mediated by phototropin is initiated at lower fluence rates than that mediated by UVR8 (Vanhaelewyn et al. 2016b). The UV-B-induced phototropic response involves the establishment of an auxin gradient across the hypocotyl, as in the UV-A/blue light response, but formation of the gradient in UV-B does not require phototropins and involves some different auxin signaling components to phototropism mediated by UV-A/blue light (Vandenbussche et al. 2014). UVR8 mediates repression of genes involved in auxin biosynthesis and signaling, which likely contributes to the generation of the auxin gradient across the hypocotyl. Vandenbussche & Van Der Straeten (2014) showed that the accumulation of HY5 on the UV-B exposed side of the hypocotyl (demonstrated using a HY5-YFP fusion) correlated with UVR8 response kinetics and is likely to mediate the repression of auxin biosynthesis genes on the illuminated side.
A further response involving UVR8 and auxin signaling is leaf epinasty, which is the downward curling of leaf edges away from incident light. Epinasty is stimulated by UV-B exposure (Wilson & Greenberg 1993; Jansen 2002) and also by the action of phyB, whereas phototropins promote leaf flattening (Kozuka et al. 2013). Fierro et al. (2015) showed that the epinastic response to UV-B in Arabidopsis is mediated by UVR8, most likely through the regulation of auxin transport. Moreover, they found considerable overlap in the sets of genes regulated by UVR8 and phyB, notably in the repression of genes involved in auxin action. The phyB action in epinasty involves the regulation of specific PIFs (Johansson & Hughes 2014), and there is evidence that PIFs are required for the UV-B-induced response (Fierro et al. 2015). A possible scenario is that UV-B de-stabilizes PIFs, as in the inhibition of shade avoidance, causing the repression of auxin response genes and consequently initiating the changes in auxin transport associated with the epinastic response.
Fasano et al. (2014) highlighted the potential interactions between UVR8 and abiotic stress signaling pathways and proposed that the cross-talk may involve auxin signaling. They reported that high salt and osmotic stress stimulate UVR8 expression and that a uvr8 mutant has increased salt tolerance under UV-B conditions. In addition, the reduced extension growth of plants over-expressing UVR8, previously observed by Favory et al. (2009), was enhanced under osmotic stress. Fasano et al. (2014) found that the UVR8 over-expression phenotype is due to reduced cell expansion and suggested that the phenotype could be explained by altered auxin signaling. Abiotic stresses such as drought, salinity and high temperature will often be accompanied by relatively high fluence rates of UV-B in nature, and the interplay between UVR8 signaling and auxin signaling could be modulated under such conditions to regulate growth and promote survival.
The stimulation of stomatal closure by UV-B involves interaction of UVR8 with different signaling pathways to those that regulate growth responses. In species such as Vicia faba (Jansen & Noort 2000) and Arabidopsis (Eisinger et al. 2003; He et al. 2013; Tossi et al. 2014), low fluence rates of UV-B stimulate stomatal opening whereas higher fluence rates promote closure. He et al. (2013) showed that the closure response in Arabidopsis is mediated by an increase in H2O2, generated through NADPH oxidase activity. UV-B-induced cytosolic alkalinization is involved in mediating the increase in H2O2 production (Zhu et al. 2014). In turn H2O2 stimulates NO production (He et al. 2013). Inhibition of endogenous NO accumulation prevents closure even under conditions where H2O2 remains high (Tossi et al. 2014). Tossi et al. (2014) found that UV-B-induced stomatal closure is impaired in uvr8, with a concomitant reduction in H2O2 and NO accumulation in the guard cells. Nevertheless, the mutant stomata were viable, and they closed when either a NO donor or abscisic acid was added. It is likely that UVR8 acts to promote H2O2 and hence NO accumulation, but it is not clear how it does so. The UVR8 action likely involves gene expression, because a mutant lacking the HY5/HYH transcription factors is impaired in the closure response (Tossi et al. 2014), but the relevant target genes are not known.
The ability of UVR8 to influence auxin and gibberellic acid signaling, as well as redox signaling, is likely to affect a larger number of physiological processes than reported to date. Furthermore, it is likely that interactions between UVR8 and additional signaling pathways will be discovered. UVR8 photoreception leads to sequestration of COP1 and stimulation of HY5 accumulation, and both these proteins participate in a range of cellular processes (Lau & Deng 2012; Huang et al. 2014a; Gangappa & Botto 2016). For instance, COP1 is involved in controlling abundance of the flowering time regulator CONSTANS (Jang et al. 2008; Liu et al. 2008; Sarid-Krebs et al. 2015), and hence UVR8 activation might influence flowering time, as suggested in some studies (Morales et al. 2013; Fasano et al. 2014). HY5 binds to over 9000 genomic loci in Arabidopsis (Zhang et al. 2011) and regulates genes in numerous processes (Gangappa & Botto 2016). Thus, regulation of HY5 provides a potential mechanism for UVR8 to influence several aspects of plant physiology. Figure 3 illustrates some of the known and potential interactions involving UVR8.
8 likes
comments
Share
Used techniques
LST
Technique
6
Week 6. Flowering8mo ago
12 hrs
Light Schedule
29 °C
Day Air Temp
50 %
Air Humidity
24 °C
Night Air Temp
Nutrients 6

Microbes Bloom Stage
5.21 mll

RAW Cane Molasses
5.21 mll

RAW Amino Acids
0.65 mll
Ultraviolet Things of this world are in so constant a flux, that nothing remains long in the same state.
20 lbs of black lava rock was added as mulch, raising soil temperature to around 1 and a half degrees to 72.8F. Some nice little bud formations are creeping up already. Applying a foliar spray of some aminos to the underside of the leaf. Hard to gauge or know how much the aminos help, but after reading how energy-intensive it is for the plant to make them from scratch, it's something I feel I need to do as a habit. Enzymes, too, don't like higher temperatures; they denature. Every little helps. Not much, just a couple sprays in the afternoon at UVB peak.
An EC (Electrical Conductivity) meter, one that's made for the soil, it's so useful, as it indirectly indicates soil moisture as well as salt mineral nutrient levels. Just pop your metre stick in the soil and if the EC is low, then it's time to water. Once there is water to assist in the conduction of electricity, the EC" will kick back up. 0.3-1.8, if it stays low, then you know it's time to add more mineral salt ferts! While Electrical Conductivity primarily indicates the overall salt content in soil, pH provides information about the relative proportion of cations (positively charged ions) in the soil's salt capacity. High EC signifies a higher salt concentration, while pH reflects the balance of cations like calcium, magnesium, potassium, ammoniacal nitrogen, sodium, and hydrogen.
Sugars, classified as carbohydrates, are composed of the elements carbon (C), hydrogen (H), and oxygen (O). They are characterized by the general formula (CH2O)n, where 'n' represents the number of carbon atoms. The most basic units of sugars, called monosaccharides, have this ratio of carbon, hydrogen, and oxygen. For example, glucose and fructose, both monosaccharides, have the formula C6H12O6.
The reality of your typical plant. After harvest, 90% of the entire plant is water, with all the water removed, you are left with. (Ballpark)
Mother-nutrients: Carbon 47%, Oxygen 43%, Hydrogen 4%.
Macro-nutrients: Nitrogen 3%, Phosphorus1%, Potassium1%, Calcium1%, Magnesium0.5%, Sulfur0.5%.
Micro-nutrients: All the rest combined 1%
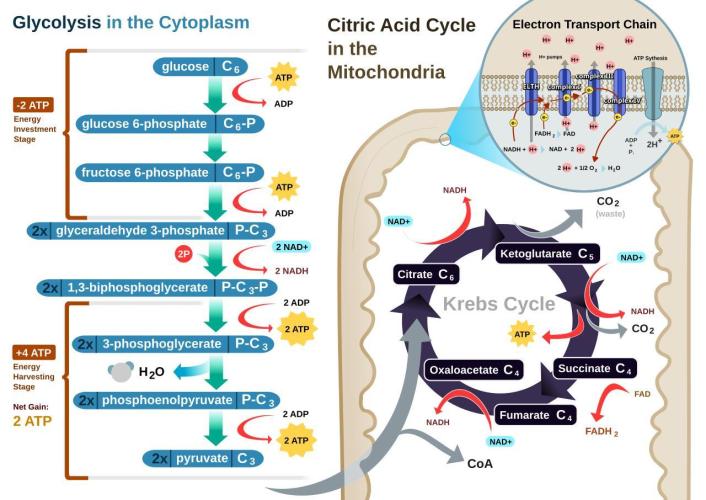
Glycolysis:
Microorganisms can break down sugars into their constituent atoms, though they don't typically do so completely to the individual elemental level (carbon, hydrogen, oxygen) in one step. Microorganisms utilize sugars through metabolic pathways like glycolysis and fermentation, converting them into simpler molecules like pyruvate and then potentially to other compounds like lactic acid, ethanol, or carbon dioxide, releasing energy in the process. This is a central pathway where a glucose molecule (a common sugar) is broken down into two molecules of pyruvate. This process generates some ATP (energy) for the cell. If oxygen is limited, some microorganisms can ferment pyruvate, producing various end products like lactic acid (in lactic acid fermentation), ethanol and carbon dioxide (in alcoholic fermentation), or other organic acids. The products of glycolysis and fermentation can be further broken down through other metabolic pathways, potentially leading to the release of carbon dioxide and water, and the extraction of more energy. While some microorganisms can completely oxidize sugars to carbon dioxide and water, releasing all their energy, others may stop at intermediate stages, producing various organic compounds. Microorganisms use specific enzymes to catalyze each step in these breakdown pathways. In summary, while microorganisms don't typically reduce sugars to individual atoms in one go, they break them down into simpler molecules, releasing energy and potentially forming new compounds as part of their metabolism.
In conditions of high CO2 concentration, the pH of a solution or system will decrease, becoming more acidic. Conversely, low CO2 concentrations lead to an increase in pH, making the solution more alkaline or basic. This relationship is due to the chemical reactions involving CO2 and water, which produce carbonic acid and influence the concentration of hydrogen ions, ultimately determining the pH. Soil respiration releases both carbon dioxide (CO2) and some nitrogen compounds, including nitrates (NO3), back into the atmosphere.
Ultra Tip:
Soil "breathing" is the key process by which soil microorganisms and plant roots release CO2 as they break down organic matter. Changes in atmospheric pressure will cause air to move in and out of the soil, impacting the diffusion of gases like carbon dioxide (CO2), which is a byproduct of soil respiration. By adding carbon sugars, the microorganisms feed and release CO2, which gets trapped in the soil. The soil is made to respire using negative pressure linked to rh% overnight. Combined with cellular respiration, CO2 is a by-product overnight. Before adding sugars, the CO2 compensation point was around 1200ppm. The first night after adding mollasees, she was spitting upwards of 1900ppm in the same timeframe. Remember, medium won't respire unless you force it out by altering the air pressure from low to high through negative pressure. The art lies in extracting the RH to create the negative pressure but not sucking so much that you take the co2 with it. Density is a beautiful thing. * When CO2 levels are high, either in the atmosphere or within the leaf (intercellular CO2), plants don't need to open their stomata as wide to obtain enough CO2 for photosynthesis. By closing their stomata, plants can reduce water loss through transpiration, which is especially important in dry or water-stressed conditions. Stomatal movements are regulated by various factors, including CO2 concentration and the plant hormone abscisic acid (ABA). ABA plays a role in stomatal closure, and elevated CO2 can affect stomatal responses through ABA signaling pathways. Not sure it would have much effect on a 5 or 7 gallon pot but certainly 4 of them.
*Please don't mess with CO2 unless you have an air quality reader of some sort, as levels above 2000 ppm with prolonged exposure can cause problems with cognitive function in enclosed spaces and its not too far from being toxic, so please don't attempt unless you have fresh air exchange in room containing the tent and a fairly adult understanding of whats going on and the potential risks. A confined space is a fully or partially enclosed area that is not designed for continuous human occupancy and has limited or restricted means of entry or exit, or an internal configuration that can complicate rescue or emergency response. Essentially, it's a space where hazards can arise due to its structure, location, contents, or the work being done within it.
47% Carbon, 43% Oxygen. Plants??? Pfffff, they are more like sugar balloons filled with salt water.
11 likes
comments
Share
7
Week 7. Flowering7mo ago
12 hrs
Light Schedule
29 °C
Day Air Temp
6.7
pH
900 PPM
TDS
50 %
Air Humidity
23 °C
Substrate Temp
24 °C
Night Air Temp
Nutrients 4
Agave Nectar
2.6 mll

RAW Cane Molasses
2.6 mll

RAW Amino Acids
0.65 mll
Ultraviolet Education begins the gentleman, but reading, good company and reflection must finish him.
Yellow butterfly came to see me the other day; that was nice.
Plant hormones and phytohormones are the same thing. Phytohormones is just another name for plant hormones, with "phyto-" meaning "plant" in Greek.
Starting to show signs of stress on the odd leaf, localized isolated blips, blemishes, who said growing up was going to be easy!
Smaller leaves have less surface area for stomata to occupy, so the stomata are packed more densely to maintain adequate gas exchange. Smaller leaves might have higher stomatal density to compensate for their smaller size, potentially maximizing carbon uptake and minimizing water loss. Environmental conditions like light intensity and water availability can influence stomatal density, and these factors can affect leaf size as well. Leaf development involves cell division and expansion, and stomatal differentiation is sensitive to these processes. In essence, the smaller leaf size can lead to a higher stomatal density due to the constraints of available space and the need to optimize gas exchange for photosynthesis and transpiration. In the long term, UV-B radiation can lead to more complex changes in stomatal morphology, including effects on both stomatal density and size, potentially impacting carbon sequestration and water use. In essence, UV-B can be a double-edged sword for stomata: It can induce stomatal closure and potentially reduce stomatal size, but it may also trigger an increase in stomatal density as a compensatory mechanism. It is generally more efficient for gas exchange to have smaller leaves with a higher stomatal density, rather than large leaves with lower stomatal density. This is because smaller stomata can facilitate faster gas exchange due to shorter diffusion pathways, even though they may have the same total pore area as fewer, larger stomata. Leaf size tends to decrease in colder climates to reduce heat loss, while larger leaves are more common in warmer, humid environments. Plants in arid regions often develop smaller leaves with a thicker cuticle and/or hairs to minimize water loss through transpiration. Conversely, plants in wet environments may have larger leaves and drip tips to facilitate water runoff. Leaf size and shape can vary based on light availability. For example, leaves in shaded areas may be larger and thinner to maximize light absorption. Leaf mass per area (LMA) can be higher in stressful environments with limited nutrients, indicating a greater investment in structural components for protection and critical resource conservation. Wind speed, humidity, and soil conditions can also influence leaf morphology, leading to variations in leaf shape, size, and surface characteristics. Small leaves: Reduce water loss in arid or cold climates.
Environmental conditions significantly affect gene expression in plants. Plants are sessile organisms, meaning they cannot move to escape unfavorable conditions, so they rely on gene expression to adapt to their surroundings. Environmental factors like light, temperature, water, and nutrient availability can trigger changes in gene expression, allowing plants to respond to and survive in diverse environments.
Depending on the environment a young seedling encounters, the developmental program following seed germination could be skotomorphogenesis in the dark or photomorphogenesis in the light. Light signals are interpreted by a repertoire of photoreceptors followed by sophisticated gene expression networks, eventually resulting in developmental changes. The expression and functions of photoreceptors and key signaling molecules are highly coordinated and regulated at multiple levels of the central dogma in molecular biology. Light activates gene expression through the actions of positive transcriptional regulators and the relaxation of chromatin by histone acetylation. Small regulatory RNAs help attenuate the expression of light-responsive genes. Alternative splicing, protein phosphorylation/dephosphorylation, the formation of diverse transcriptional complexes, and selective protein degradation all contribute to proteome diversity and change the functions of individual proteins.
Photomorphogenesis, the light-driven developmental changes in plants, significantly impacts gene expression. It involves a cascade of events where light signals, perceived by photoreceptors, trigger changes in gene expression patterns, ultimately leading to the development of a plant in response to its light environment.
Genes are expressed, not dictated! While having the potential to encode proteins, genes are not automatically and constantly active. Instead, their expression (the process of turning them into proteins) is carefully regulated by the cell, responding to internal and external signals. This means that genes can be "turned on" or "turned off," and the level of expression can be adjusted, depending on the cell's needs and the surrounding environment. In plants, genes are not simply "on" or "off" but rather their expression is carefully regulated based on various factors, including the cell type, developmental stage, and environmental conditions. This means that while all cells in a plant contain the same genetic information (the same genes), different cells will express different subsets of those genes at different times. This regulation is crucial for the proper functioning and development of the plant.
When a green plant is exposed to red light, much of the red light is absorbed, but some is also reflected back. The reflected red light, along with any blue light reflected from other parts of the plant, can be perceived by our eyes as purple.
Carotenoids absorb light in blue-green region of the visible spectrum, complementing chlorophyll's absorption in the red region. They safeguard the photosynthetic machinery from excessive light by activating singlet oxygen, an oxidant formed during photosynthesis. Carotenoids also quench triplet chlorophyll, which can negatively affect photosynthesis, and scavenge reactive oxygen species (ROS) that can damage cellular proteins. Additionally, carotenoid derivatives signal plant development and responses to environmental cues. They serve as precursors for the biosynthesis of phytohormones such as abscisic acid () and strigolactones (SLs). These pigments are responsible for the orange, red, and yellow hues of fruits and vegetables, while acting as free scavengers to protect plants during photosynthesis.
Singlet oxygen (¹O₂) is an electronically excited state of molecular oxygen (O₂). Singlet oxygen is produced as a byproduct during photosynthesis, primarily within the photosystem II (PSII) reaction center and light-harvesting antenna complex. This occurs when excess energy from excited chlorophyll molecules is transferred to molecular oxygen. While singlet oxygen can cause oxidative damage, plants have mechanisms to manage its production and mitigate its harmful effects.
Singlet oxygen (¹O₂) is considered a reactive oxygen species (ROS). It's a form of oxygen with higher energy and reactivity compared to the more common triplet oxygen found in its ground state. Singlet oxygen is generated both in biological systems, such as during photosynthesis in plants, and in cellular processes, and through chemical and photochemical reactions. While singlet oxygen is a ROS, it's important to note that it differs from other ROS like superoxide (O₂⁻), hydrogen peroxide (H₂O₂), and hydroxyl radicals (OH) in its formation, reactivity, and specific biological roles. Non-photochemical quenching (NPQ) protects plants from damage caused by reactive oxygen species (ROS) by dissipating excess light energy as heat. This process reduces the overexcitation of photosynthetic pigments, which can lead to the production of ROS, thus mitigating the potential for photodamage. Zeaxanthin, a carotenoid pigment, plays a crucial role in photoprotection in plants by both enhancing non-photochemical quenching (NPQ) and scavenging reactive oxygen species (ROS). In high-light conditions, zeaxanthin is synthesized from violaxanthin through the xanthophyll cycle, and this zeaxanthin then facilitates heat dissipation of excess light energy (NPQ) and quenches harmful ROS.
The Issue of Singlet Oxygen!!
ROS Formation: Blue light, with its higher energy photons, can promote the formation of reactive oxygen species (ROS), including singlet oxygen, within the plant.
Potential Damage: High levels of ROS can damage cellular components, including proteins, lipids, and DNA, potentially impacting plant health and productivity.
Balancing Act: A balanced spectrum of light, including both blue and red light, is crucial for mitigating the harmful effects of excessive blue light and promoting optimal plant growth and stress tolerance.
The Importance of Red Light:
Red light (especially far-red) can help to mitigate the negative effects of excessive blue light by:
Balancing the Photoreceptor Response: Red light can influence the activity of photoreceptors like phytochrome, which are involved in regulating plant responses to different light wavelengths.
Enhancing Antioxidant Production: Red and blue light can stimulate the production of antioxidants, which help to neutralize ROS and protect the plant from oxidative damage.
Optimizing Photosynthesis: Red light is efficiently used in photosynthesis, and its combination with blue light can lead to increased photosynthetic efficiency and biomass production.
In controlled environments like greenhouses and vertical farms, optimizing the ratio of blue and red light is a key strategy for promoting healthy plant growth and yield.
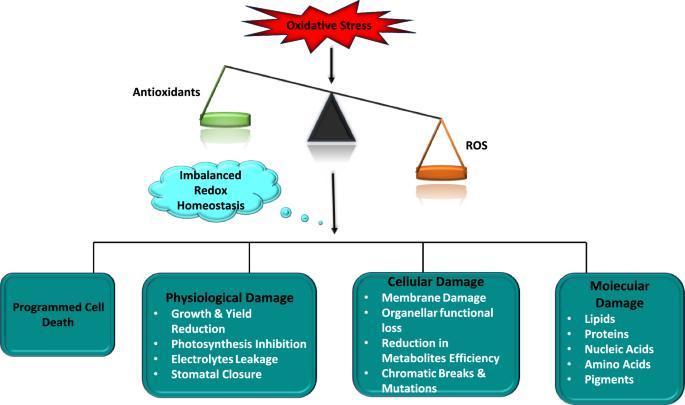
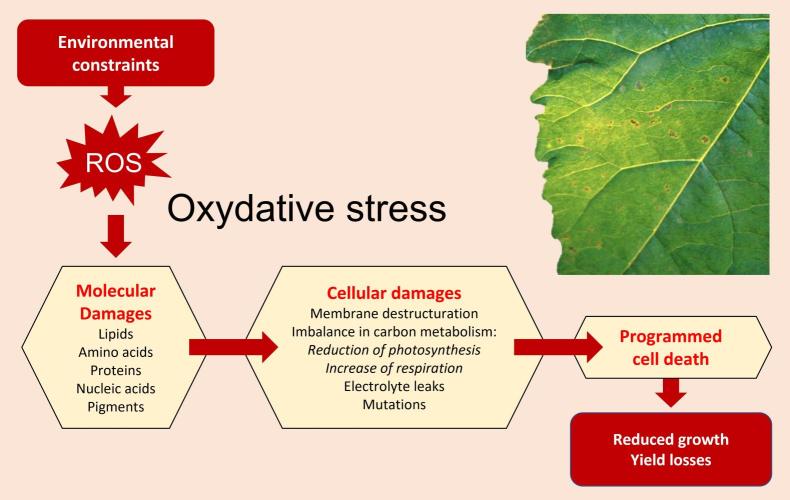
Understanding the interplay between blue light signaling, ROS production, and antioxidant defense mechanisms can inform breeding programs and biotechnological interventions aimed at improving plant stress resistance. In summary, while blue light is essential for plant development and photosynthesis, it's crucial to balance it with other light wavelengths, particularly red light, to prevent excessive ROS formation and promote overall plant health. Oxidative damage in plants occurs when there's an imbalance between the production of reactive oxygen species (ROS) and the plant's ability to neutralize them, leading to cellular damage. This imbalance, known as oxidative stress, can result from various environmental stressors, affecting plant growth, development, and overall productivity.
Causes of Oxidative Damage: Abiotic stresses: These include extreme temperatures (heat and cold), drought, salinity, heavy metal toxicity, and excessive light. Biotic stresses: Pathogen attacks and insect infestations can also trigger oxidative stress. Metabolic processes: Normal cellular activities, particularly in chloroplasts, mitochondria, and peroxisomes, can generate ROS as byproducts. Certain chlorophyll biosynthesis intermediates can produce singlet oxygen (1O2), a potent ROS, leading to oxidative damage. ROS can damage lipids (lipid peroxidation), proteins, carbohydrates, and nucleic acids (DNA). Oxidative stress can compromise the integrity of cell membranes, affecting their function and permeability. Oxidative damage can interfere with essential cellular functions, including photosynthesis, respiration, and signal transduction. In severe cases, oxidative stress can trigger programmed cell death (apoptosis). Oxidative damage can lead to stunted growth, reduced biomass, and lower crop yields.
Plants have evolved intricate antioxidant defense systems to counteract oxidative stress. These include: Enzymes like superoxide dismutase (SOD), catalase (CAT), and various peroxidases scavenge ROS and neutralize their damaging effects. Antioxidant molecules like glutathione, ascorbic acid (vitamin C), C60 fullerene, and carotenoids directly neutralize ROS. Developing plant varieties with gene expression focused on enhanced antioxidant capacity and stress tolerance is crucial. Optimizing irrigation, fertilization, and other management practices can help minimize stress and oxidative damage. Applying antioxidant compounds or elicitors can help plants cope with oxidative stress. Introducing genes for enhanced antioxidant enzymes or stress-related proteins over generations.
Phytohormones, also known as plant hormones, are a group of naturally occurring organic compounds that regulate plant growth, development, and various physiological processes. The five major classes of phytohormones are: auxins, gibberellins, cytokinins, ethylene, and abscisic acid. In addition to these, other phytohormones like brassinosteroids, jasmonates, and salicylates also play significant roles.
Here's a breakdown of the key phytohormones:
Auxins: Primarily involved in cell elongation, root initiation, and apical dominance.
Gibberellins: Promote stem elongation, seed germination, and flowering.
Cytokinins: Stimulate cell division and differentiation, and delay leaf senescence.
Ethylene: Regulates fruit ripening, leaf abscission, and senescence.
Abscisic acid (ABA): Plays a role in seed dormancy, stomatal closure, and stress responses.
Brassinosteroids: Involved in cell elongation, division, and stress responses.
Jasmonates: Regulate plant defense against pathogens and herbivores, as well as other processes.
Salicylic acid: Plays a role in plant defense against pathogens.
1. Red and Far-Red Light (Phytochromes):
Red light:
Primarily activates the phytochrome system, converting it to its active form (Pfr), which promotes processes like stem elongation and flowering.
Far-red light:
Inhibits the phytochrome system by converting the active Pfr form back to the inactive Pr form. This can trigger shade avoidance responses and inhibit germination.
Phytohormones:
Red and far-red light regulate phytohormones like auxin and gibberellins, which are involved in stem elongation and other growth processes.
2. Blue Light (Cryptochromes and Phototropins):
Blue light:
Activates cryptochromes and phototropins, which are involved in various processes like stomatal opening, seedling de-etiolation, and phototropism (growth towards light).
Phytohormones:
Blue light affects auxin levels, influencing stem growth, and also impacts other phytohormones involved in these processes.
Example:
Blue light can promote vegetative growth and can interact with red light to promote flowering.
3. UV-B Light (UV-B Receptors):
UV-B light:
Perceived by UVR8 receptors, it can affect plant growth and development and has roles in stress responses, like UV protection.
Phytohormones:
UV-B light can influence phytohormones involved in stress responses, potentially affecting growth and development.
4. Other Colors:
Green light:
Plants are generally less sensitive to green light, as chlorophyll reflects it.
Other wavelengths:
While less studied, other wavelengths can also influence plant growth and development through interactions with different photoreceptors and phytohormones.
Key Points:
Cross-Signaling:
Plants often experience a mix of light wavelengths, leading to complex interactions between different photoreceptors and phytohormones.
Species Variability:
The precise effects of light color on phytohormones can vary between different plant species.
Hormonal Interactions:
Phytohormones don't act in isolation; their interactions and interplay with other phytohormones and environmental signals are critical for plant responses.
The spectral ratio of light (the composition of different colors of light) significantly influences a plant's hormonal balance. Different wavelengths of light are perceived by specific photoreceptors in plants, which in turn regulate the production and activity of various plant hormones (phytohormones). These hormones then control a wide range of developmental processes.
9 likes
1 comment
Share
8
Week 8. Flowering7mo ago
12 hrs
Light Schedule
31 °C
Day Air Temp
6.5
pH
800 PPM
TDS
50 %
Air Humidity
23 °C
Substrate Temp
25 °C
Night Air Temp
Nutrients 4

Bud Factor X
2 mll

RAW Cane Molasses
0.65 mll

RAW Bloom
1.3 mll
Ultraviolet The discipline of desire is the background of character.
Discipline can be described as the act of doing what one does not want to do, especially when it's necessary for achieving a desired outcome or fulfilling a commitment. It involves overcoming personal resistance, laziness, or lack of motivation to pursue a goal or adhere to a standard. Essentially, it's about prioritizing long-term benefits over immediate desires or discomfort.
Why do I run the line with high EC even when my tips are showing signs of osmotic damage?
While soil electrical conductivity (EC) primarily indicates the concentration of dissolved salts and ions, making it a measure of electrical capacity, it is also a crucial indicator of potential life processes within the soil. EC levels reflect nutrient availability, water-holding capacity, and the overall health of the soil ecosystem, influencing microbial activity and plant growth. As soil moisture drops, so too does its EC, regardless of its salt mineral level/capacity. Keeping the foot on the pedal, not letting that EC ever drop below 1.0 in the medium. Low EC, Low life processes capacity. Microorganisms can create and utilize electrical currents, a process known as extracellular electron transfer (EET), which plays a role in various phenomena, including microbial corrosion and the development of microbial electrochemical technologies (METs). This EET is facilitated by microbial biofilms that develop on conductive surfaces, allowing for electron exchange. ATP is created when a phosphate group is added to ADP (adenosine diphosphate), and this process often involves energy derived from the breakdown of glucose during cellular respiration. Water plays a crucial role in the breakdown of ATP (hydrolysis) to release energy, but it's not the source of that energy. In essence, water is involved in both ATP synthesis (in a roundabout way through proton gradients) and ATP breakdown (hydrolysis). However, water itself is not the source of energy for ATP synthesis; rather, it's the breakdown of glucose and other molecules that provides the energy for ATP production.
A key part of cellular respiration(predominantly nighttime process) is oxidative phosphorylation, where a proton gradient is created across a membrane, and this gradient drives the synthesis of ATP by an enzyme called ATP synthase. Doesn't matter how much light you harvest if you don't process any of the captured carbon energy into actual usable energy (ATP)? Oxidative phosphorylation is a metabolic pathway that uses the energy released from the transfer of electrons (from NADH and FADH2) to oxygen to produce ATP (adenosine triphosphate), the cell's main energy currency. It occurs in the mitochondria and is a crucial part of cellular respiration, particularly in aerobic(oxygen-loving) organisms. This process involves two main components: the electron transport chain and chemiosmosis.
In essence, low EC can signal either: Low water content: Not enough water to carry the necessary ions or Low salt (ion) concentration: Insufficient amounts of dissolved ions in the solution.
If EC is low, ATP potential is low. Electrical conductivity is a measure of how easily electrical current can flow through a medium. It quantifies a material's ability to conduct electricity, indicating how readily electrons can move through it. A higher conductivity value means the material is a better conductor, while a lower value indicates it's a poorer conductor. What are we conducting? Salt ion concentration. The more capacity for the flow of materials for life in the form of electrical signals to pass through the medium. Electrical conductivity (EC) in a nutrient solution is a key indicator of nutrient availability and plays a crucial role in determining nutrient uptake efficiency by plants. While EC doesn't directly dictate nutrient uptake, it influences it indirectly by affecting the concentration of dissolved nutrients and the solution's osmotic potential.
While plant roots don't directly use electricity in the way we think of electrical devices, they do utilize electrochemical gradients and active transport (Transport that's uses energy) to uptake water and nutrients. These processes involve the movement of ions across cell membranes, which can be influenced by electrical potential differences.
1. Electrochemical Gradients: Plant roots have specialized proteins embedded in their cell membranes (like H+-ATPases) that act as channels and transporters for ions.
Establishing Gradients: These proteins actively pump ions across the membrane, creating concentration gradients and electrical potential differences (voltage).
Passive Transport: Ions can then move passively down these gradients, either into or out of the cell, depending on the specific ion and its concentration.
Example: H+-ATPases pump protons (H+) out of the cell, creating a proton gradient. This gradient can then be used to bring other ions like nitrate or phosphate into the cell, even against their concentration gradient.
2. Active Transport:
ATP as Energy Source: Active transport requires the cell to expend energy, often in the form of ATP, to move ions against their concentration gradient.
Specific Transporters: Proteins called carriers are involved in active transport, binding to specific ions and facilitating their movement across the membrane. Uptake of potassium (K+), nitrate (NO3-), and phosphate (PO43-) often involves active transport.
3. Water Uptake:
Osmosis: Water uptake is primarily driven by osmosis, where water moves from an area of high water potential (low solute concentration) to an area of low water potential (high solute concentration).
Electrical Signals and Water Uptake: Plant water uptake creates electrical signals (self-potential) due to the movement of water and ions in the soil around the roots.
Impact of Salinity: High salt concentrations can affect root conductivity and potentially lead to toxicity. Electrical stimulation can be used to enhance nutrient uptake by activating root hair formation and ion transport.
Gene expression, the process by which information from a gene is used to synthesize a functional gene product (like a protein), is significantly influenced by nutrient availability. Cells constantly monitor nutrient levels and adjust their gene expression patterns to optimize growth, survival, and function in response to these fluctuating conditions.
High nutrient availability can significantly influence plant growth regulators (PGRs), impacting various aspects of plant development. Nutrient levels affect hormone synthesis, transport, and signaling pathways, leading to changes in root and shoot growth, branching, and overall plant architecture. (PGRs are essentially phytohormones, same thing).
Water uptake in plants is primarily dictated by osmosis and transpiration pull, which are influenced by factors like soil water potential, root hydraulic conductivity, and stomatal regulation. Osmosis drives water movement from the soil into root cells, while transpiration, a consequence of water evaporation from leaves, creates a negative pressure gradient that draws water up the plant.
More transpiration =more pull, more cohesion, more pressure to angle leaves into satellite "Disciplined" fashion, more root penetration = Higher rate of water uptake.
More EC = Higher concentration of salt mineral uptake within the water that's being taken at a given rate.
Light, Energy capacity in 1 cycle 40-60DLI.
Temperature, 82.8 Leaf surface temp,(not ambient) metabolic optimal.
Humidity, optimize VPD for the stage of growth. Nightime: 45-55% Never above, never below.
CO2, not utilized by temperature.
Wind: (air flow), at least some ambient air flow, with at least 78 cfm (4x4) extraction linked to RH for negative pressure. The more she spits out, the more she turns on.
Water: Unlimited EC=to or greater than 1.0
Nutrients: Unlimited EC =to or greater than 1.0
Root-zone temperature: Optimal 72.8°F
Oxygen in the root system: Unlimited. 55% pore space + Negative pressure diffusion.
PH 6.2-6.7 While oxygen uptake is more directly influenced by soil aeration and water content, soil pH also plays a role.
Changes in air pressure, including those created by high and low pressure fronts(negative pressure), can influence soil respiration. Fluctuations in atmospheric pressure cause air movement in and out of the soil, a phenomenon known as "barometric pumping," which can affect the rate at which soil releases gases like carbon dioxide and nitrogen.
Maintaining a pH within the optimal range for phosphorus availability (6.0-7.0) generally also favors good soil structure and aeration, which are important for oxygen uptake by plant roots. Extremes of pH (either very acidic or very alkaline) can lead to poor soil structure, hindering root growth and oxygen diffusion.
Neglecting any one of these can significantly impact a plant's well-being. (There is no such thing as too much water, only a medium that retains too much for too long. By making your environment slightly drier, slightly warmer, you encourage plant metabolism, optimize photosynthetic capture efficiency, increase nutrient uptake through transpirational increase, and remove the possibility of overwatering by encouraging evaporation and cellular respiration.
Root respiration is directly reliant on cellular respiration. Root respiration is the process where plant roots take up oxygen and release carbon dioxide, and this process is fueled by the energy produced during cellular respiration. Cellular respiration breaks down glucose to produce ATP, which powers various cellular activities, including those in the root cells that facilitate nutrient and water uptake.
Increased atmospheric CO2 concentration can be detrimental to plant growth under certain conditions, even though CO2 is necessary for photosynthesis. While elevated CO2 can stimulate photosynthesis, it also triggers stomatal closure, reducing water loss but potentially limiting CO2 uptake if temperatures are not high enough to support the increased metabolic demand. This can lead to a situation where the plant's ability to utilize the increased CO2 is hampered, resulting in actual reduced growth. While increased CO2 can boost photosynthetic rates, this effect is most pronounced at optimal temperatures. If temperatures are not high enough to support the increased metabolic demand from higher CO2 levels, the reduced stomatal aperture can limit CO2 uptake and water loss, thus hindering photosynthesis and overall growth. If temperatures are not optimal for the increased CO2 concentration, plants may experience reduced photosynthetic rates, impaired growth, and decreased biomass production.
Optimal VPD (Vapor Pressure Deficit) and optimal plant respiration conditions are not the same, though they are related. VPD is primarily concerned with the rate of transpiration, which impacts water and nutrient uptake, while respiration is the process of energy production within the plant. Different VPD levels can affect stomatal opening, influencing photosynthesis and potentially impacting respiration indirectly. Optimal vapor pressure deficit (VPD) conditions, while beneficial for transpiration and overall plant health, do not directly enhance cellular respiration. Cellular respiration, the process of converting sugars into energy, is primarily influenced by factors like temperature and enzyme activity, not VPD directly. While VPD affects stomatal behavior and water movement, which indirectly impact photosynthesis and carbon availability, it doesn't directly drive the biochemical reactions of cellular respiration
Respiration rates are highly influenced by temperature. Generally, respiration increases with temperature up to a certain point (around 50°C), after which it decreases due to damage to plant tissues.
While VPD can indirectly affect respiration by influencing stomatal conductance and photosynthesis, its direct impact is primarily on transpiration and water relations. VPD affects stomatal behavior, which in turn influences gas exchange (CO2 uptake for photosynthesis and oxygen release). Photosynthesis is linked to respiration, as the products of photosynthesis (sugars) are used in cellular respiration. In essence, while VPD is a crucial factor in plant water relations and photosynthesis, its impact on cellular respiration (nighttime) is indirect and less significant compared to temperature and substrate availability.
•Optimize for VPD during the day, optimize cellular respiration by night; what is good for vpd is not always good for respiration.
•Water runs low, and nothing good ever happens. (Except maybe drought stress, but now is not the time)
•Water sits in any one place too long, nothing good ever happens.
•Oxygen runs low in the soil, nothing good ever happens.
• CO2 without the temperature to assist with metabolic pathways, nothing too good, potentially detrimental.
Without a corresponding increase in temperature, plants cannot fully benefit from higher levels of CO2. Photosynthesis, the process by which plants convert CO2 and light into energy, is temperature-dependent. Therefore, while elevated CO2 can enhance photosynthesis under optimal temperature conditions, it won't be as effective if the temperature isn't also suitable. I'm aware of this. If this were a photoperiod, temps would be in the 91-92°F ambient. I know what to expect from high temperatures, high light. I want to see the difference with controlled temps. 🤔 Gave her a few nights in the 60's to see how low I could get her for ripening later. Worried the summer heat would prevent lower than 70F, but all is well. Back to keeping her around 77-80 overnight for now until trichomes ramp up,
Time to apply Bud Factor X, produced by Advanced Nutrients, is a product that triggers a plant's natural defense mechanism called Induced Systemic Resistance (ISR). This process involves the plant releasing proteins and enzymes to combat external threats like pests and pathogens. Bud Factor X achieves this by mimicking natural molecules that the plant uses to trigger ISR, without actually causing stress or damage to the plant. This leads to increased essential oil production, improved plant health, and enhanced resistance to environmental stressors. This one will already be frosty, so I'm excited to apply. I made the hint earlier that these would be frosty as I noticed very early pre-flowers had more trichomes than I'm used to seeing, she hadn't even started bud formation, and she was cranking out non-glandular trichomes. It's still very early, but yeah, can see the frosty coating already, going to be something else entirely come harvest. Administer Bud Factor X at a rate of 2ml per litre of nutrient solution. Bud Factor X can also be applied as a foliar spray, though it's recommended that this is undertaken before the formation of flowers to prevent issues with excess humidity and fungal infections.
9 likes
1 comment
Share
9
Week 9. Flowering7mo ago
12 hrs
Light Schedule
29 °C
Day Air Temp
650 PPM
TDS
50 %
Air Humidity
23 °C
Substrate Temp
25 °C
Night Air Temp
Nutrients 3

Bud Factor X
2 mll

RAW Cane Molasses
0.65 mll

RAW Bloom
1.3 mll
Ultraviolet No man's knowledge here can go beyond his experience.
ATP, or adenosine triphosphate, is created through several cellular processes. The main pathways include glycolysis, the citric acid cycle (also known as the Krebs cycle), and oxidative phosphorylation. Additionally, ATP can be generated through substrate-level phosphorylation, which occurs during glycolysis and the citric acid cycle, and through beta-oxidation of fatty acids.
1. Cellular Respiration:
Glycolysis:
This is the initial stage of glucose breakdown, occurring in the cytoplasm. It produces a small amount of ATP (2 molecules per glucose) and NADH, an electron carrier.
Citric Acid Cycle (Krebs Cycle):
Pyruvate, produced during glycolysis, is converted into acetyl-CoA, which enters the citric acid cycle in the mitochondria. This cycle generates more ATP (2 per glucose) and electron carriers (NADH and FADH2).
Oxidative Phosphorylation:
This process occurs in the mitochondria and utilizes the electron carriers (NADH and FADH2) generated in the previous stages to create a proton gradient across the mitochondrial membrane. This gradient then drives ATP synthase to produce a large amount of ATP (around 26-28 per glucose). A large percentage, roughly 90%, of ATP (adenosine triphosphate), the cell's primary energy currency, is produced within the mitochondria. This is primarily achieved through a process called oxidative phosphorylation, which is part of cellular respiration and occurs in the mitochondria's inner membrane. This is a nightime process. High RH% will severly cripple this process. Vpd is for transpiration. Transpiration is a majority daytime process.
The potential difference between these two redox pairs is 1.14 volt, which is equivalent to -52 kcal/mol or -2600 kJ per 6 mol of O2. When one NADH is oxidized through the electron transfer chain, three ATPs are produced, which is equivalent to 7.3 kcal/mol x 3 = 21.9 kcal/mol.
Mitochondria are often called the "powerhouses of the cell" because they are the primary sites for ATP production. This is the main pathway for ATP synthesis in mitochondria. It involves the electron transport chain and chemiosmosis, which work together to generate a proton gradient and ultimately produce ATP.
Oxidative phosphorylation is a key part of aerobic respiration, which uses oxygen to generate ATP from glucose.
Aerobic respiration, with its reliance on mitochondria, is significantly more efficient than anaerobic processes, such as fermentation, in terms of ATP yield. For example, aerobic respiration can produce around 30 ATP molecules per glucose molecule, while fermentation only yields 2 ATP.
Aerobic and anaerobic respiration are two processes cells use to produce energy (ATP), but they differ in their requirement for oxygen. Aerobic respiration requires oxygen, while anaerobic respiration does not. Aerobic respiration is more efficient, producing significantly more ATP per glucose molecule, but it's a slower process. Anaerobic respiration produces less ATP but can occur rapidly when oxygen is limited.
2. Substrate-Level Phosphorylation:
This process involves the direct transfer of a phosphate group from a substrate molecule to ADP, forming ATP.
It occurs during glycolysis and the citric acid cycle, contributing to the initial ATP production in these pathways.
3. Beta-Oxidation:
This pathway breaks down fatty acids into acetyl-CoA, which then enters the citric acid cycle, contributing to ATP production.
4. Photosynthesis (in plants):
Plants use light energy to convert carbon dioxide and water into glucose during photosynthesis. This process also generates ATP, which is then used to fuel the synthesis of glucose.
ATP Biosynthesis
In essence, ATP is generated through a combination of these pathways, with cellular respiration (glycolysis, citric acid cycle, and oxidative phosphorylation) being the primary mechanism in most organisms.
While all are essential, ATP is fundamentally the most critical for plant growth as it directly fuels cellular processes, with nutrients, oxygen, and carbon playing secondary but equally vital roles.
Elaboration:
1. ATP as the Energy Currency:
ATP (adenosine triphosphate) is the primary energy carrier in cells, including plant cells. It powers various cellular activities like nutrient uptake, protein synthesis, and cell division. Without ATP, the plant's metabolic machinery would grind to a halt, regardless of the presence of nutrients, oxygen, or carbon.
2. Nutrients as Building Blocks:
Nutrients, like nitrogen, phosphorus, and potassium, are essential for building plant tissues and various molecules. They are incorporated into proteins, nucleic acids, and other vital compounds. While crucial, their uptake and utilization rely on ATP-driven processes.
3. Oxygen for ATP Production:
Oxygen is vital for cellular respiration, a process that generates ATP. While plants can produce ATP through photosynthesis, oxygen is essential for maximizing ATP production in mitochondria through oxidative phosphorylation.
4. Carbon as the Primary Component:
Carbon is the backbone of all organic molecules, including carbohydrates synthesized during photosynthesis. It's the fundamental building block of plant structures and fuels. However, its incorporation into organic molecules is also ATP-dependent.
Will electrolysis increase the concentration of hydrogen in water? In a nutshell, yes, but 99% of it will escape as a gas, so until you can figure out a way to capture it and recombine it with the water, while simultaneously eliminating all oxygen production, but therin lies the problem, as soon as you start to try to capture gases, the concentrations become volatile very quickly, simply can not "mess" with pressurizing hydrogen in anyway without serious risk. Oxygen is always just 2% away from spontaneous combustion.
For context, 90% of a plant is water. After 100% of that water is removed, you have approximately:
47%carbon,
43%oxygen,
4%hydrogen,
3%nitrogen,
1%phosphorous,
1%pottasium,
1%calcium ,
.5%magnesium,
.5%sulfur,
all the rest 1%
Water (H2O) is composed of two hydrogen atoms and one oxygen atom. While there are two hydrogen atoms, the oxygen atom's larger mass (16 times heavier than hydrogen) means it contributes the majority (88.89%) of the water's mass.
The concentration of dissolved hydrogen gas (H2) in water is very, very low, typically around 8.65 x 10^-7 mg/L.
Hydrogen tablets can increase hydrogen concentration. I'm not so interested in seeing if I can process more water rather than creating a huge surplus of energy.
Plants obtain the hydrogen they use to build organic molecules from splitting water molecules during photosynthesis.
4% Hydrogen, with the symbol H and atomic number 1, is the first element on the periodic table. While "H" in hydrogen is fundamentally a chemical symbol for the element, hydrogen, in its molecular form (H2), has shown promise in various health-related applications. This is largely due to its antioxidant and anti-inflammatory properties, potentially offering protection against oxidative stress and related diseases.
How it works, a plant's ability to handle light is limited by the potential for ROS (reactive oxygen species) oxidation. When plants are exposed to excessive light, they can produce damaging ROS molecules, particularly in the chloroplasts during photosynthesis. This excess ROS can lead to photo-oxidative stress, potentially damaging cellular components like lipids, proteins, and DNA, and ultimately affecting plant health and survival. Higher hydrogen content, particularly in the form of reduced compounds like NADPH or hydrogen radicals, can play a protective role against oxidative stress caused by high light intensities in photosynthetic organisms.
This protection arises because these hydrogen-containing molecules can effectively scavenge reactive oxygen species (ROS), such as superoxide radicals and hydrogen peroxide, which are generated under high light and can damage cellular components.
The apple skin is packed full of hydrogen to prevent the apple body from being oxidized. As soon as you take a bite, it goes brown within 30 minutes.
More light exposure leads to increased oxidation, and a higher hydrogen content can enable a plant to better handle increased light exposure. hydrogen can act as an antioxidant, mitigating the damaging effects of high light stress on the plant's photosynthetic machinery.
High EC forces the plant to utilize active ion transport, not passive.
For every molecule of glucose produced during photosynthesis, a plant needs to split six molecules of water. This process provides the hydrogen needed for synthesizing glucose and other organic compounds, while oxygen is released as a byproduct.
The overall equation for photosynthesis is 6CO2 + 6H2O + Light energy → C6H12O6 + 6O2. During the light-dependent reactions of photosynthesis, water molecules are split (photolysis) to release electrons, hydrogen ions (H+), and oxygen. The released hydrogen ions (H+) and electrons are crucial for reducing carbon dioxide (CO2) to form glucose (C6H12O6), which is a simple sugar and the main energy source for the plant. The oxygen (O2) released during water splitting is a byproduct of this process and is released into the atmosphere.
Everything is energy, energy in motion, e-motion. All inventions are built upon existing knowledge and observations of the natural world.
"I have not failed 10,000 times. I've just found 10,000 ways that won't work."
11 likes
10 comments
Share
10
Week 10. Flowering7mo ago
12 hrs
Light Schedule
25 °C
Day Air Temp
300 PPM
TDS
50 %
Air Humidity
23 °C
Substrate Temp
24 °C
Night Air Temp
Nutrients 1

RAW Cane Molasses
2.6 mll
Ultraviolet We are like chameleons; we take our hue and the color of our moral character from those who are around us.
A fundamental principle in ecology. Growth in nature is dictated by the availability of energy. Living organisms need energy to perform all life processes, including growth, reproduction, and maintenance. The amount and type of energy available directly influence the rate and extent of that growth.
Although the plant does create some ATP during photosynthesis and "carbon capture" , 90% of all the usable energy each day/night cycle will be processed during cellular respiration, converting the carbon captured into actual usable energy. Cellular respiration occurs continuously, 24/7, in all living organisms to convert glucose into energy. It is not limited to day or night and happens independently of photosynthesis, which only occurs in plants during daylight.
VPD is about pressure differences between water membranes, optimizing stomatal conductance for efficient movement/cohesion of water through the plant. That process is called transpiration, and it creates turgor pressure built from the corresponding osmosis, forming a cohesive column of water across membranes and through the plant.
Transpiration, although it can occur at night if it gets hot, without light there is no endothermic energy to drive the gas exchange, VPD is 10x less effective at night because there is little to no transpiration at night, the plant mainly utilizes daytime transpiration 90% water use, as a method of passively uptaking nutrients for free "Bulk flow" (without a ATP energy cost). Doesnt mean a plant cannot uptake nutrients at night, just means it comes with an energy cost attached. (Active Transport).
Cellular respiration doesn't use water from the root zones; it just splits what it exchanges through respiration. Carbon is captured, being broken down using oxygen as a catalyst. Cellular respiration in cannabis plant roots is reliant on oxygen both in the root zone (soil) and in the air. While roots absorb oxygen from the soil through diffusion, the availability of oxygen in the air directly impacts the root zone's oxygen levels and the overall efficiency of cellular respiration. 40% of the carbon that a plant captures is exuded into the root zones as sugars to feed the microorganisms. A lot of the CO2 gets trapped in the soil as the microorganisms break it down. By running a negative pressure linked to rh, it creates a rhythmic diffusion of oxygen "into" and of CO2/Nitrogen gas out of the soil and into the air. In nature, this would be mimicking high and low-pressure fronts.
By adding extra sugar/carbon in the form of molasses to the medium, the micros get supercharged, releasing far more CO2 into the tent overnight than you would normally get with cellular respiration alone. If you set up your fans correctly, you can extract humidity and keep the dense CO2 overnight for morning consumption. When daylight comes, I turn on the vertical floor fan. Kick all that CO2 up there.
Added a 700-1000nm infrared light overnight to increase temps and help encourage a little more co-enzymes that promote cellular respiration within the mitochondria. Autoflowers are useful.
It's a plant.
It's an energy processing plant.
Input and output efficiency are similar concepts. If the input efficiency increases, you need fewer inputs for the same output; and if your output efficiency increases, you gain more output for the same input. Throughput can be thought of as "size of the factory". A larger throughput means more output, but also the same increase in input. We load the medium with inputs, set the environment that dictates the rate of output, and this creates a throughput of water. 90% of water uptake during the day is cooling-related, and 10% of the water is split during photosynthesis for creating around 10% of total ATP production during daytime photosynthesis. The hydrogen from water is also scavenged and used to protect the plant from its mortal enemy, singlet oxygen free radicals. The plant can only handle as much excess light as it is able to protect itself from these free radicals. Singlet oxygen is highly reactive, and if the production of ROS (Reactive oxygen species) exceeds the plant's ability to deal with/neutralize them, then acids and other nasties will form that break apart proteins, fats, and lipids, eventually until PCD kicks in.
The metabolic pathways of photosynthesis are influenced by both light intensity and temperature. Light intensity directly affects the light-dependent reactions, while temperature impacts the light-independent or (Calvin cycle) reactions, which are enzyme-catalyzed. Once heat is removed as a factor, light and/or the amount it can use is only limited by the plant's ability to deal with or suppress the radicals; the plant gets its hydrogen from splitting water.
Not advisable to supplement CO2 above 1200ppm without using temperature to activate the metabolic pathways otherwise, it can potentially do more to hinder growth than promote it, I don't think many people are prepared for the amount of water that gets used when you start to run 90's in your tent, I couldn't make it to the tent frequent the enough to keep 7 gallon pots wet enough long enough, part of the reason I eventually got the 100 gallon. Plants transpire 3x at 68F than at 86F, with added airflow and increases in temps, you need to run 91F ambient to hold an internal leaf surface of 86F (that's @ 400 ppm) optimal for photosynthesis photon capture.
All that extra water splitting and hydrogen to protect from photo-oxidation, all that extra carbon capture as potential energy..................you will never see even a fraction of that potential processed into actual usable energy with low temps and high RH overnight. A fundamental principle in ecology. Growth in nature is dictated by the availability of energy. Although a leaf can perform both process day or night a optimized vpd for transpiration at high temps is not good at all for cellular respiration.
Principle, "Principle indicates something being first or most important."
Cellular respiration relies on enzymes to catalyze biochemical reactions. Enzymes have optimal temperature ranges for their activity. At low temperatures, enzyme activity slows down, reducing the rate of respiration. As temperature increases, enzyme activity generally increases up to a certain point, leading to a higher rate of respiration. However, excessively high temperatures can denature enzymes, causing them to lose their function and halting cellular respiration.
Besides temperature, other factors like substrate concentration (e.g., sucrose, glucose) and oxygen availability (especially for aerobic respiration) also play crucial roles in determining the rate of cellular respiration.
I've been monitoring my tent's CO2 for years now, down low, up high, in flow, out flows, all over the tent. CO2 is created at night as a by-product of cellular respiration occurring both from the plant and the microorganisms in the soil through root respiration. Think of CO2; it's more like plant exhaust fumes. It gives a solid indication of the amount of work being done, or "how much cellular respiration occurred." The more CO2 in my tent overnight, the more carbon has been converted into usable ATP. For example last few days, I've reached 1600-1800ppm by morning. The next day, I checked she was 500ppm. Something was up, nothing got converted that night. Pot had ran a little drier than I expected, almost wasted the entire cycle of growth. It's an interesting tool I can use to make sure there is always a good level of "work" being done at critical times in development.
Cellular respiration is the fundamental metabolic process that occurs in all living plant cells (roots, stems, leaves, flowers, seeds) to convert glucose into usable energy (ATP). It requires oxygen and produces carbon dioxide and water as byproducts.
Root respiration is not a separate type of respiration, but rather a specific instance of cellular respiration occurring in the roots. Roots absorb oxygen from the air spaces in the soil to fuel their energy needs.
Roots require a substantial amount of ATP for essential functions like nutrient and water absorption, as well as growth and maintenance of their tissues. The primary form of respiration in healthy plant roots is aerobic respiration, which is highly efficient, yielding up to 30-38 ATP molecules per glucose molecule. If roots are deprived of oxygen (e.g., in waterlogged soil), they switch to anaerobic respiration, which is far less efficient, producing only 2 ATP molecules per glucose molecule and also generating alcohol, which can be toxic and kill the plant. Therefore, a plant with healthy, oxygenated roots performing efficient aerobic respiration in addition to the rest of the plant's cellular respiration has a much greater overall ATP production and energy capacity.
7 likes
4 comments
Share
11
Week 11. Flowering6mo ago
25 °C
Day Air Temp
450 PPM
TDS
45 %
Air Humidity
20 °C
Night Air Temp
Nutrients 3

RAW Calcium / Mag
0.65 mll

RAW Bloom
1.3 mll

RAW Cane Molasses
0.65 mll
Ultraviolet All men are liable to error; and most men are, in many points, by passion or interest, under temptation to it.
54DLI @ 12 hours
The stem that snapped clean off in week 5, the resulting stress seems to have made a 2ft solid massive cola, probably the biggest individual I've grown, albeit she had signs of stress, and I felt like she was a little behind in the floral development compared to others, but as the rest of the plants start to settle down with packing on mass, the stressed colawas just getting started. Super interesting to see the difference that the snap made to the base of the stem overall, as I know before the snap, they were all the same girth or thereabouts. Neat little lucky accident, maybe I'll try something next grow.
81 grams dry.
Start to cool things off now.
YB.
10 likes
6 comments
Share
12
Week 12. Flowering6mo ago
12 hrs
Light Schedule
23 °C
Day Air Temp
250 PPM
TDS
45 %
Air Humidity
22 °C
Substrate Temp
17 °C
Night Air Temp
Nutrients 4
Mixer
1.3 mll

RAW Amino Acids
0.65 mll

RAW Enzymes
0.65 mll
Ultraviolet We should have a great fewer disputes in the world if words were taken for what they are, the signs of our ideas only, and not for things themselves.
Fatties. Cold as I can get her for last week. Light reduced to roughly 30dli.
11 likes
comments
Share
13
Week 13. Harvest6mo ago
Happy Harvest Day!

10/10
Rated
She smells floral, piney, with very strong diesel tones that create a powerful aroma. Very clean narcotic relaxing high, was not expecting such a quality high, probably up there top 5 fave all time somewhere, zero negative side effects, especially now she has cured, she is starting to smell very potent, slightest squeeze of a nug is enough to engulf a room with the waft of pure dank.
Show more
Translate
Spent 86 days
Ger Veg Flo Har
145 g
Bud dry weight per plant
4
Plants
10.16 m²
Grow Room size
Easy
Difficulty

Relaxed
Positive effects
Day air temperature
Air humidity
PPM
PH
Light schedule
Night air temperature
Substrate temperature
Ultraviolet What can be made to expand, airy and loose, can also be made to contract, dense and tight. Buds are primarily composed of water. Developing flower buds, like other plant tissues, require a significant amount of water for growth and turgor pressure, which helps maintain their structure and firmness. Turgor pressure in plant cells is primarily generated by osmosis, but transpiration plays a crucial role in maintaining it.
25 likes
9 comments
Share
Equipment Reviews

the end.
Enjoying this diary? Follow for more updates!
Prefer the old Diary view?
Go back to the old Diary view